Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.101 n.10 Pretoria Oct. 2011
SCIENTIFIC LETTERS
Treatment of paediatric burns with a nanocrystalline silver dressing compared with standard wound care in a burns unit: a cost analysis
S G CoxI; L CullingworthII; H RodeIII
IMB ChB, FCS (SA), Cert Paed Surg (SA). Department of Paediatric Surgery, University of Cape Town and Red Cross War Memorial Children's Hospital, Cape Town
IIMB ChB, DFSRH (UK), MPH (Health Economics). 3 Degree Clinical Research and Consulting
IIIMB ChB, MMed (Surg), FRCS (Edin), FCS (SA). Department of Paediatric Surgery, Red Cross War Memorial Children's Hospital
ABSTRACT
Burns are a leading cause of non-natural death in South African infants and children. Conventional care of partial-thickness burns often requires painful, time consuming and costly twice-daily dressing changes to clean the wound and apply antimicrobial topical agents. A new topical nanocrystalline silver-coated (NS) dressing (Acticoat; Smith & Nephew) has been developed and is the first-line treatment of choice in many burn centres. However, because of its cost the Department of Health has been reluctant to introduce it as a standard of care. We retrospectively studied 4 randomly selected paediatric burn patients, calculating the cost associated with the use of NS dressings and comparing this with the projected costs of three previously standard burn wound treatment regimens. NS dressings were changed every 3 days based on their sustained and slow release of silver ions over 72 hours. Using NS clearly saved costs compared with the three other regimens. The demonstrated cost savings resulted primarily from the decreased number of dressings, and the presumed shorter hospital stay.
To the Editor: Burns are the leading cause of non-natural death in infants and children aged under 5 years and the fourth major cause of accidental death in the 5 - 9-year age group,1 and more than 1 300 children die annually from burns in South Africa.
Since the treatment of burn wounds is among the most resourceintensive of all paediatric trauma care,2 cost-effective treatment without compromising the clinical outcome is needed in the South African public health sector.
Conventional care of partial-thickness burns often requires twicedaily dressing changes to clean the wound and apply antimicrobial topical agents.3-5 These are often painful, time consuming and costly. New topical agents have been developed to target the increasing inefficiency of current agents, and the emergence of new and resistant organisms. Silver inactivates almost all bacteria and many fungi and extracellular viruses, and favourably influences mortality.4,6 However, silver-containing topical agents have become less effective,7 which negatively impacts on patient morbidity and septic mortality.
To overcome resistance patterns, a topical nanocrystalline silvercoated dressing containing Ag+, Ag0 and additional silver ions (Acticoat; Smith & Nephew) (NS) was developed. Studies6,8 confirmed its antibacterial spectrum and anti-inflammatory properties.9 In many burn centres NS is the first-line treatment of choice and has changed the management of small to moderate-sized burns from a largely inpatient to an outpatient process.10 However, the Department of Health has been reluctant to introduce this as a standard of care, as it costs more than other 'traditional' dressings.
A study comparing the effectiveness and cost of Silvazine (silver sulphadiazine and chlorhexidine digluconate cream) and NS in the inpatient treatment of early burn wounds found that the average length of stay (LOS) in hospital was 40% shorter in the NS group, which also had a total cost saving of approximately 39%.11 Others have confirmed the antimicrobial properties12 and cost-effectiveness of NS.10
In 2004 NS was introduced into the Red Cross War Memorial Children's Hospital burns unit as the preferred treatment for moderate to major partial- and full-thickness burns. Because of the potential cost implications, an audit was performed to evaluate the cost effectiveness of NS compared with standard treatment methods.
Methods
We conducted a retrospective case study with 4 randomly selected paediatric burn patients, 1 from each of the body surface area groups 20 - 29%, 30 - 39%, 40 - 49% and 50 - 59%. The cost associated with the use of NS dressings was calculated and compared with the projected costs of three previously standard burn wound treatment regimens. Only the treatment period during which patients received NS was costed. Outside of the NS treatment period the wounds were dressed with standard topical dressings, or were operated on. NS dressings were changed every 3 days based on their sustained and slow release of silver ions over 72 hours. Other topical agents would have required daily dressing changes to retain their maximal phamacodynamic and antiseptic efficacy, and the cost analysis was conducted according to this requirement.
The cost analysis was performed using the ingredients approach, and was performed from the public sector perspective. Input costs reflect prices for the 2009 financial year and expressed in South African rands (ZAR).
NS costs were calculated based on patients being taken to theatre for all dressing changes (standard practice in our unit for pain control, optimisation of sterility and ease of dressing changes for major burns). For standard burn therapy (SBT), we assumed that: (i) length of stay (LOS) for patients receiving SBT is 38% longer than for those receiving NS dressings;11 and (ii) patients receiving SBT require a minimum of a third of their dressing changes to be performed in theatre.
The following resource categories were taken into consideration: (i) theatre fees as per the Uniform Patient Fee Schedule (UPFS) categories (UPFS 2009 Western Cape, revised June 2009, Annexure A, available from: http://www.doh.gov.za/programmes/upfs/docs/2009/tariff_2009.pdf) ; (ii) ward fees as per the UPFS categories (UPFS 2009 Western Cape); and (iii) medical supplies for topical burn care therapy as per Western Cape 2009 tender prices (or where not on tender, the price ex-manufacturer). All prices were inclusive of valueadded tax (VAT) at 14%.
To effectively compare costs, the cost of three alternative treatment regimes for SBT for a burn area of 20×40 cm (the size that one portion of the product covers) was calculated (listed below). Since 150 g of the topical agent is required to effectively cover a burned area of 20×40 cm, the costs were calculated as follows: (i) tulle gras (Jelonet; Smith & Nephew) + mupirocin + chlorhexidine, R134.95 (i.e. R5.20 + R109.79 + R19.96); (ii) tulle gras + silver sulphadiazine, R17.90 (i.e. R5.20 + R12.70); and (iii) tulle gras + povidone iodine (5%), R9.94 (i.e. R5.20 + R4.74).
As the burns unit laboratory investigations and feeding of patients are standardised, these were not costed. Drugs, human resources, overhead, capital and indirect costs were not included.
Results
The four patients studied were 2 boys and 2 girls, aged 1 - 12 years. Injuries were due to fire in 3 cases and hot water in 1, resulting in deep partial- to full-thickness burns of 21%, 45%, 33%, and 53% body surface area, respectively. Patient injury details and treatment duration are set out in Table I.
Table II and Fig. 1 compare actual theatre-based NS costs with calculated SBT costs, and show the cost saving when using NS compared with SBT.
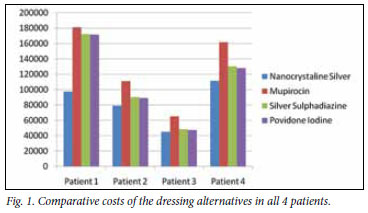
NS versus mupirocin/chlorhexidine mixture on a jelonet carrier: Average cost (R129 700) was calculated as 1.6 times the cost of using NS (R83 316) - average cost saving of R46 383 per patient.
NS versus silver sulphadiazine 1% cream on a jelonet carrier: Average cost (R110 341) was calculated as 1.3 times the cost of using NS (R83 316) - average cost saving of R27 024 per patient.
NS versus povidone iodine 5% cream on a jelonet carrier: Average cost (R108 999) was calculated as being 1.3 times the cost of using NS (R83 316) - average cost saving of R25 683 per patient.
In terms of safety, no patient developed any local or systemic reaction to NS.
Discussion
The care of children with burns is a resource-intensive process posing particular challenges in terms of pain control, frequent cleansing of wounds and the application of topical antibacterial agents to avoid infection.
Many topical agents are used for burn care. They are not universally effective, and differ in their ability to penetrate an eschar and in their bio- and antibacterial activity and side-effects.3 They may be combined to enhance their effects. A single agent that consolidates most of the desired activities of a topical agent would be beneficial in terms of frequency and ease of application, be effective, not have undesirable side-effects, and be cost-effective and reduce the development of resistant patterns. NS dressings combines most of these beneficial effects in a single dressing.11 The dressing was approved by the US Food and Drug Administration as a barrier dressing to prevent and treat burn wound sepsis.9 Silver (Ag0) also has anti-inflammatory activity independent of antimicrobial action, induces apoptosis in inflammatory cells, and suppresses matrix metalloproteinase activity and pro-inflammatory cytokines.9 Because of these factors, commercial availability and increasing use of NS, a practical cost assessment had to be made.
Using NS clearly saves costs compared with three other regimens of standard burn therapy. A major advantage of this dressing is that it only needs to be changed every 3 days. The need in 'standard' burn care for daily dressing changes increases the burden of burn wound care and pain, nosocomial wound infection risk, costs of care and risk of damaging the newly formed epithelial skin layer.
The demonstrated cost savings resulted primarily from the decreased number of dressings, and the presumed shorter hospital stay. Likely further cost drivers include the increased use of antiinfectives with SBT,11 and increased human resource costs; however, these resource categories were not studied. NS is not recommended as a dressing for donor sites or for small superficial burns that can be treated effectively with standard treatment modalities.13
While acknowledging that this is a very small selection of patients and larger numbers are required for more accurate comparison, this study is an example of how management that is perceived to be prohibitively expensive for the state health service may be more cost effective and clinically appropriate than originally anticipated.
References
1. Prinsloo M, Seedat M, Kotzenberg C. A profile of fatal injuries in South Africa Seventh Annual Report of the National Injury Mortality Surveilance System 2005: MRC/UNISA Crime, Violence and injury Lead Programme. http://www.sahealthinfo.org/violence/national2005.pdf (accessed May 2010). [ Links ]
2. Esselman PC, Ptacek T, Kowalske K, et al. Community integration after burn injuries. J Burn Care Rehabil 2001;22(3):221-227. [ Links ]
3. Ward RS, Saffle JR, Topical agents in burn and wound care. Phys Ther 1995;75(6):526-538. [ Links ]
4. Wright JB, Lam K, Burrell RE. Wound management in an era of increasing bacterial antibiotic resistance: a role for topical silver treatment. Am J Infect Control 1998;26(6):572-577. [ Links ]
5. Lukish JR, Eichelberger MR, Newman KD, et al. The use of a bioactive skin substitute decreases length of stay for pediatric burn patients. J Pediatr Surg 2001;36(8):1118-1121. [ Links ]
6. Wright JB, Lam K, Hansen D, et al. Efficacy of topical silver against fungal burn wound pathogens. Am J Infect Control 1999;27(4):344-350. [ Links ]
7. Klasen HJ. A historical review of the use of silver in the treatment of burns. II. Renewed interest for silver. Burns 2000;26(2):131-138. [ Links ]
8. Tredget EE, Shankowsky H A, Groeneveld A, et al. A matched-pair, randomized study evaluating the efficacy and safety of Acticoat silver-coated dressing for the treatment of burn wounds. J Burn Care Rehabil 1998;19(6):531-537. [ Links ]
9. Wright JB, Lam K, Buret AG, et al. Early healing events in a porcine model of contaminated wounds: effects of nanocrystalline silver on matrix metalloproteinases, cell apoptosis, and healing. Wound Repair Regen 2002;10(3):141-151. [ Links ]
10. Cuttle L, Naidu S, Mill J, et al. A retrospective cohort study of Acticoat versus Silvazine in a paediatric population. Burns 2007;33(6):701-707. [ Links ]
11. Fong J, Wood F, Fowler B. A silver coated dressing reduces the incidence of early burn wound cellulitis and associated costs of inpatient treatment: comparative patient care audits. Burns 2005;31(5):562-567. [ Links ]
12. Yin HQ, Langford R, Burrell RE, Comparative evaluation of the antimicrobial activity of ACTICOAT antimicrobial barrier dressing. J Burn Care Rehabil 1999;20(3):195-200. [ Links ]
13. Innes ME, Umraw N, Fish JS, et al. The use of silver coated dressings on donor site wounds: a prospective, controlled matched pair study. Burns 2001;27(6):621-627. [ Links ]
Accepted 16 May 2011.
Corresponding author: S Cox (sharon.cox@uct.ac.za)














