Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.101 n.9 Pretoria Sep. 2011
ORIGINAL ARTICLES
Potential impact of reactive vaccination in controllingcholera outbreaks: an exploratory analysis using a Zimbabwean experience
Sun-Young KimI; Yeongchull ChoiIII; Peter R MasonIV; Simbarashe RusakanikoIV; Sue J GoldieII
IPhD. Center for Health Decision Science, Department of Health Policy and Management, Harvard School of Public Health, Boston, Massachusetts, USA, and Harvard Global Health Institute, Harvard University, Cambridge, Massachusetts, USA
IIMD, MPH. Center for Health Decision Science, Department of Health Policy and Management, Harvard School of Public Health, Boston, Massachusetts, USA, and Harvard Global Health Institute, Harvard University, Cambridge, Massachusetts, USA
IIIMD, MPH, MBA. Department of Health Policy and Management, Harvard School of Public Health, Boston, Massachusetts, USA
IVPhD. Biomedical Research and Training Institute and University of Zimbabwe College of Health ciences, Harare, Zimbabwe
ABSTRACT
BACKGROUND: To contain ongoing cholera outbreaks, the World Health Organization has suggested that reactive vaccination should be considered in addition to its previous control measures.
OBJECTIVES: To explore the potential impact of a hypothetical reactive oral cholera vaccination using the example of the recent large-scale cholera outbreak in Zimbabwe. Methods. This was a retrospective cost-effectiveness analysis calculating the health and economic burden of the cholera outbreak in Zimbabwe with and without reactive vaccination. The primary outcome measure was incremental cost per disability-adjusted life year (DALY) averted.
RESULTS: Under the base-case assumptions (assuming 50% coverage among individuals aged >2 years), reactive vaccination could have averted 1 320 deaths and 23 650 DALYs. Considering herd immunity, the corresponding values would have been 2 920 deaths and 52 360 DALYs averted. The total vaccination costs would have been ~$74 million and ~$21 million, respectively, with per-dose vaccine price of US$5 and $1. The incremental costs per DALY averted of reactive vaccination were $2 770 and $370, respectively, for vaccine price set at $5 and $1. Assuming herd immunity, the corresponding cost was $980 with vaccine price of $5, and the programme was cost-saving with a vaccine price of $1. Results were most sensitive to case-fatality rate, per-dose vaccine price, and the size of the outbreak.
CONCLUSION: Reactive vaccination has the potential to be a costeffective measure to contain cholera outbreaks in countries at high risk. However, the feasibility of implementation should be further evaluated, and caution is warranted in extrapolating the findings to different settings in the absence of other in-depth studies.
Cholera is preventable by providing clean water and adequate sanitation, hygiene education, food safety, oral cholera vaccines, etc., and readily treatable through oral rehydration therapy (ORT).1,2 It is endemic in many countries in Africa and South Asia, where several minor and major outbreaks have been reported.3
A large-scale cholera outbreak in Zimbabwe demonstrated how a preventable and easily treatable enteric disease can have disastrous outcomes that can be exacerbated by a weak health system infrastructure, sub-optimal availability of and access to basic water/sanitation, and lack of political will to improve the system. Between August 2008 and July 2009, 98 592 cases of cholera reportedly occurred in Zimbabwe alone, leading to 4 288 deaths (case fatality rate (CFR) 4.3%).4 The worst cholera outbreak affected all provinces in Zimbabwe and spread to South Africa, Mozambique, Zambia, Angola and Malawi.5
The Zimbabwean outbreak prompted a rethink about the most effective strategy to prevent and contain cholera outbreaks.6 This, together with changes in epidemiological trends of cholera and the availability of a new vaccine, resulted in the World Health Organization (WHO) issuing a new position paper concerning cholera outbreaks. The mainstay of control measures should be to provide appropriate treatment and improve water and sanitation, and community mobilisation. However, the WHO suggests that pre-emptive vaccination may play a role in preventing or containing outbreaks. The WHO also suggests that 'reactive vaccination could be considered by local health authorities as an additional measure, depending on the local infrastructure and following a thorough investigation of the current and historical epidemiological situation, and clear identification of geographical areas to be targeted'.7 Nevertheless, the GAVI Alliance (a public-private partnership to increase access to vaccines in the poorest countries) has not prioritised support for cholera vaccines in developing countries before at least 2013.8
Few studies have examined the value of cholera vaccines in controlling cholera outbreaks. To provide stakeholders and policy makers with information on the potential value of oral cholera vaccines and to provoke discussion, we used the Zimbabwe outbreak to explore the cost-effectiveness of a hypothetical reactive vaccination from the global society perspective.
Methods
Base-case scenario for reactive vaccination using oral cholera vaccines
It is reported that: (i) the first case of the recent cholera outbreak in Zimbabwe occurred in mid-August 2008; (ii) the cholera outbreak was detected in September 2008; (iii) after its detection the Zimbabwe Health Cluster developed an operational plan to control the outbreak;9 (iv) during the outbreak, the international community (United Nations (UN), WHO, governments, non-governmental organisations (NGOs), and other donors) worked with the Zimbabwean Ministry of Health and Child Welfare to control the epidemic, including the establishment of a Cholera Command and Control Centre to co-ordinate responses and delivery of medicine and health equipment to health centers;10 (v) during the crisis, because of the health systems breakdown in Zimbabwe, most cholera patients were treated at cholera treatment centres (CTCs) or cholera treatment units (CTUs) that were temporarily opened across the country and mostly operated by NGOs such as Médecins Sans Frontières;11 and (vi) despite the large scale of the humanitarian response, by the end of July 2009, 98 952 cases and 4 288 deaths were recorded.
We therefore hypothetically assumed that: (i) a reactive vaccination had been planned as an additional measure to contain the cholera outbreak during its early stage; (ii) a reactive mass vaccination campaign had been conducted starting from November 2008, and approximately 50% of the target population (individuals aged >2 years, N=12 782 00012) had received two doses (7 - 14 days apart) of vaccine by the second week of December 2008; and (iii) protective efficacy had been achieved among all effectively vaccinated individuals by mid-December 2008, as cholera vaccines require about a week after the last dose administration to reach protective efficacy. Regarding the natural history of cholera and vaccine characteristics, we assumed that: (i) ~15% of reported (symptomatic) cholera events are severe cases2 requiring admission to medical facilities or CTCs/CTUs, and the remainder are mild to moderate, allowing outpatient treatment; (ii) the vaccine has 84% efficacy against symptomatic cholera (regardless of severity) and death;13 and (iii) the duration of protection is 6 months for children aged 2 - 4 years and 2 years for people aged >5 years.13
Decision-analytical approach
We developed a simple Excel-based model to calculate retrospectively the burden of the cholera outbreak in terms of disability-adjusted life years (DALYs) averted, with and without reactive vaccination using oral cholera vaccines (Fig. 1). We conservatively assumed that the reactive vaccination campaign would not have prevented any cholera cases or deaths that occurred before mid-December 2008 (i.e. 18 413 cases and 976 deaths4), but would have reduced portions of the cholera cases and deaths recorded between mid-December 2008 and the end of July 2009, depending on vaccine efficacy and coverage. Based on the assumed age distribution of the cholera events (caption Fig. 1) and Zimbabwe's age-specific life expectancy14 and population projection,12 the model translated the known and assumed numbers of cholera episodes and deaths with and without reactive vaccination into DALYs, following WHO recommended methods15 (Fig. 1). For resource use, we included direct medical costs (programme costs and cholera treatment costs) only. The primary outcome measure was incremental cost per DALY averted. To assess parameter uncertainty and examine the implications of alternative assumptions to the basecase, we performed comprehensive sensitivity analyses including: (i) univariate and multivariate sensitivity analyses; (ii) a threshold analysis; and (iii) a scenario analysis, by varying the values of key uncertain variables or assumptions one at a time or in combination over plausible ranges.
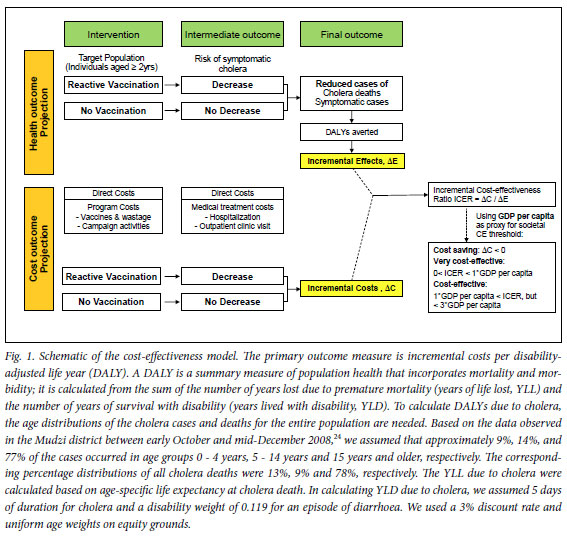
Assumptions on herd immunity effect
A crucial issue in estimating the cost-effectiveness of mass vaccination campaigns using oral cholera vaccines is whether they provide herd immunity effects and, if so, how the level of potential herd immunity depends on vaccine coverage. Studies by Ali et al.16 and Longini et al.17 suggest that mass vaccination using oral cholera vaccines can confer indirect protection to unvaccinated individuals. Based on their findings we explored the potential impact of herd immunity due to oral cholera vaccination, in one set of analysis considering herd immunity, assuming that vaccinating 50% of the Zimbabwean population would have lead to overall protection of about 93% (we relied on the findings from Bangladesh, where cholera is endemic, because of a lack of studies in settings like Zimbabwe where cholera is considered epidemic). Although the 84% vaccine efficacy assumed in our analysis is higher than that (50% for susceptibility and 70% for infectiousness for a population in Bangladesh) used in the simulation study by Longini et al.17 and may lead to a higher overall vaccine efficacy against symptomatic cholera, we conservatively assumed an overall vaccine efficacy of 93%, in part for comparability with a study that explored the implications of herd immunity18 based on the same findings.16,17
Assumptions on resource use
To estimate the resource use associated with a hypothetical reactive vaccination programme, we considered direct medical costs (vaccination programme costs and medical treatment costs) only, not including direct non-medical costs such as transportation and patient time costs. All costs were expressed in 2008 US dollars. For the vaccination programme costs, we included vaccine purchase costs and vaccine delivery costs. For the base-case analyses, we assumed per-dose prices of US$1 and $5 and a 10% vaccine wastage rate, but widely varied these assumptions in the sensitivity analysis. For vaccine delivery, we assumed US$0.50 per dose, which is often assumed for average vaccine delivery costs in low-income countries, for comparability, but varied the value up to $2.40 per dose (based on the per-child operational costs for a measles campaign in Zimbabwe19) in the sensitivity analysis. In the absence of reactive vaccination, direct medical costs were assumed to be the total costs for treating cholera cases. Estimating such costs using an ingredient approach (numbers of units used multiplied by unit cost of each item) is challenging, as no detailed data are available. Accordingly, as a proxy for cholera treatment costs, we relied on the aggregate costs associated with running CTCs or CTUs during the outbreak (Table I). The base-case medical treatment cost under no reactive vaccination was assumed to be $36 million, but was varied widely in the sensitivity analysis owing to the high parameter uncertainty, e.g. we assumed an upper limit of $131 million, which is 1.5 times greater than the humanitarian aid given by the international community for the Zimbabwe cholera crisis between 2008 and May 2009 (~$87 million, according to UN reports20,21). The medical treatment costs under reactive vaccination were based on the same approach but were assumed to decrease in proportion to the reduction in cholera cases/deaths due to vaccination (summary Table I).
Results
Base-case results
The total numbers of cholera cases and deaths reported during the Zimbabwe cholera crisis were translated into 78 240 DALYs (discounted at 3%). If reactive vaccination had been conducted approximately 2 - 3 months after the outbreak had started, under the base-case assumptions without considering herd immunity (50% coverage and 84% vaccine efficacy), approximately 32 570 cases (~33% reduction) and 1 320 deaths (~31% reduction) could have been averted, equating to approximately 23 650 DALYs averted (~30% reduction). Incorporating the potential impact of herd immunity, approximately 72 110 cases, 2 920 deaths and 52 360 DALYs could have been averted (Table II).
Under the base-case assumptions (50% coverage, $36 million of total treatment costs, and vaccine delivery cost of $0.50 per dose), if vaccine price per dose had been set at $5, the total vaccination programme costs would have been ~$74.1 million. Assuming a vaccine price of $1 per dose, the corresponding total would have been ~$20.6 million. Without reactive vaccination the total cholera treatment costs would have been ~$36.0 million. Under a reactive cholera vaccination programme the medical treatment costs would have been decreased to ~$9.8 million and ~$24.2 million, respectively, with and without considering potential herd immunity.
Combining the health and cost outcomes, without herd immunity, under the base-case assuming $5 per vaccine dose, the estimated incremental cost per DALY averted was $2 770. Assuming a vaccine price of $1 per dose, the incremental cost was substantially reduced to $370. When we assumed that reactive cholera vaccination at 50% coverage could have conferred herd immunity, the incremental cost-effectiveness ratio was $980 per DALY averted with a per-dose vaccine price set at $5, and, when we used a per-dose vaccine price of $1, the reactive vaccination programme was cost-saving (Table II).
Uncertainty analysis
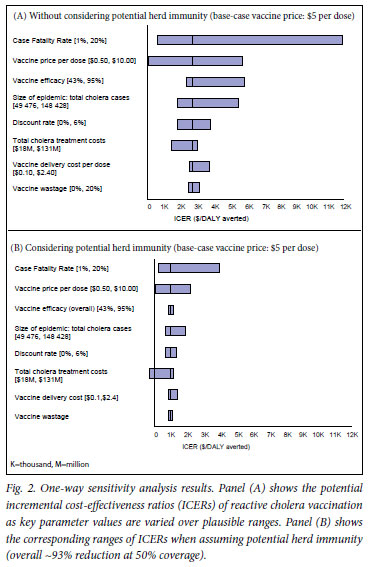
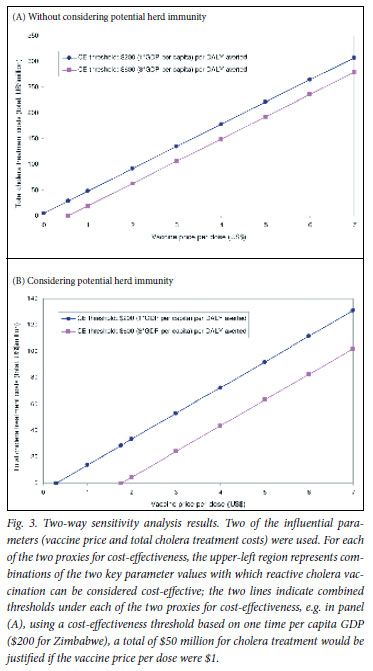
In the one-way sensitivity analysis, the results were most sensitive to CFR, per-dose vaccine price and the size of epidemic (i.e. total number of cholera cases), regardless of consideration of herd immunity (Fig. 2). Using the WHO's approach, which considers an intervention with a cost-effectiveness ratio less than the GDP per capita (~$200 for Zimbabwe) 'very cost-effective' and one with a cost-effectiveness ratio less than 3 times the GDP per capita (~$600) 'cost-effective', under the assumption of herd immunity, the breakeven values of vaccine efficacy above which reactive vaccination could be considered very cost-effective or cost-effective were 66% and 42%, respectively. Without considering herd immunity, no corresponding break-even values within the possible range (0 -100%) were identified (Table III). For vaccine price, the break-even costs for cost-saving were $0.38 and $1.40, respectively, with and without herd immunity (Table III). Fig. 3 illustrates the two-way sensitivity analysis results using two of the influential parameters, vaccine price and total cholera treatment costs. Table IV presents selected results of a scenario analysis in which a comprehensive set of key variables/assumptions were varied over plausible ranges, in combinations taking into account correlations among different variables/assumptions. These suggest that the incremental costs per DALY averted cost-effectiveness profiles of the hypothetical reactive vaccination may vary widely across scenarios.
Discussion
We show that reactive vaccination using oral cholera vaccines could have been effective and cost-effective in halting a large-scale cholera outbreak. However, these outcomes can vary widely depending on choices of parameter values and assumptions, e.g. the scale and severity of the outbreak, vaccine price, and the presence and level of herd immunity. For example, in our base-case analysis, the costeffectiveness ratios that are based on four different combinations of vaccine price and assumptions on the presence of herd immunity ranged from cost-saving to $2 770 per DALY averted. Our results are probably a conservative estimate of the impact of the reactive cholera vaccination because: (i) the reported cases and deaths are probably underestimates owing to the limited capacity to obtain accurate disease statistics, particularly during the early phase of the outbreak (we recognise that there may also be over-reporting, given that a small number were laboratory-proven while the majority were clinically diagnosed during the outbreak); (ii) we did not include the cholera burden in neighboring countries as a result of the Zimbabwe outbreak; and (iii) we did not include the health benefits that might be realised in the year after the outbreak (i.e. between August 2009 and July 2010) through reduction in cholera cases among vaccinated individuals aged >5 years while vaccine efficacy lasts.
Our study weaknesses are mainly associated with uncertainty due to data gaps and the questionable feasibility of the reactive campaign scenario of our model. We could not evaluate in depth the feasibility of achieving a high coverage rate among individuals of such a wide age range in a short time during a cholera crisis. Also, we were not able to fully explore what the epidemic outcomes would have been like if different sub-populations or geographical areas had been targeted or if the reactive vaccination had been initiated earlier or later with different scales. Owing to data gaps, we relied on rough assumptions in estimating the costs for cholera management and for a vaccination campaign. The base-case estimates of both costs might be underestimates given the narrow scopes of cost items considered (however, for vaccine cost-effectiveness considerations, underestimating both costs concurrently may have limited impact, since treatment and vaccination costs influence incremental cost-effectiveness ratios in opposite directions). To address some limitations we performed a comprehensive uncertainty analysis including threshold analysis and scenario analysis. Our findings should be carefully interpreted when considered for different settings, since some parameter values may be specific to the size and severity of the Zimbabwe outbreak and demographics (e.g. age-specific life expectancy), and epidemiology may vary by country.
The Zimbabwe cholera outbreak is considered to be over. However, the threat of another cholera outbreak remains, especially since the nearly-collapsed water/sanitation infrastructure and weak public health system that probably helped spark the last outbreak remain;22 further, the population still faces multiple crises such as hunger, high HIV/AIDS prevalence, and continued failure in economic recovery, with high unemployment and widespread poverty. The threat of cholera also looms over other vulnerable countries in Africa and other regions (recent outbreaks in Pakistan and Haiti) that face similar challenges. While in the long term improvement of water and sanitation as specified in Millennium Development Goal 7 is of paramount importance (also to reduce the burden of other diseases), given the slow progress towards improved sanitation,23 cholera vaccination may be a critically important tool to prevent or contain both endemic and epidemic cholera in the short term.
To plan and organise a reactive vaccination campaign in the early phases of a cholera outbreak is challenging, since the unfolding of an outbreak is hard to predict. Other challenges include the feasibility of stockpiling of cholera vaccines, which require a cold chain, for a large campaign, and reaching a targeted population within a short period during a crisis. Justifications for considering reactive vaccination as an additional measure to control cholera epidemics include: (i) the 5-year cumulative reported numbers of cholera cases has increased globally since 2001;3 (ii) the existing humanitarian responses to cholera crises may provide a high level of funding such that savings on humanitarian aid may largely offset the costs of reactive mass vaccination campaign in some vulnerable countries (as illustrated in our exploratory analysis); and (iii) a new lower-cost vaccine may become available with much improved logistics (i.e. no requirement for a buffer, which facilitates vaccine use under field conditions).13 Given these facts, the international community should seriously consider selective use of reactive vaccination in some vulnerable countries experiencing complex humanitarian crises.
Future studies may help policy makers refine the approach to cholera outbreak control: (i) epidemiological studies focused on its natural history (e.g. potential presence and duration of protection after natural infection) and the age and gender distribution of cholera cases/deaths could identify more effective strategies to control endemic and epidemic cholera; (ii) the development of an advanced model to better predict the outbreak and spread of cholera would be useful; (iii) more evidence on herd immunity conferred by oral cholera vaccines could help design appropriate control measures (e.g. determining effective and cost-effective level of vaccination coverage), particularly in communities where immune status has been compromised by other infections, especially HIV; (iv) as current oral cholera vaccines require two doses and WHO guidelines recommend targeting a broader age range of individuals for outbreak control, identifying effective ways to reach individuals and scale up a cholera vaccination programme should be a high priority, e.g. studying the benefits of a reactive campaign at the sub-national scale such as a province hit relatively late during a course of cholera outbreak; and (v) comprehensive evaluation of cost-effectiveness and affordability of multiple strategies, including ORT, water and sanitation system improvement, hygiene education, and pre-emptive or reactive vaccination - alone and in combination - would inform the development of a co-ordinated control strategy that is feasible and sustainable.
In conclusion, reactive vaccination has the potential to be a costeffective measure to control cholera outbreaks in some vulnerable countries at high risk. However, the feasibility of its implementation should be further evaluated, and caution is needed in transferring our findings to different settings.
Acknowledgements. The authors are very grateful to the anonymous reviewers for their extremely helpful comments on previous versions of the manuscript.
References
1. Sack DA, Sack BR, Nair GB, Siddique AK. Cholera. Lancet 2004;363:223-233. [ Links ]
2. Centers for Disease Control. Laboratory Methods for the Diagnosis of Epidemic Dysentery and Cholera. Atlanta, Ga: CDC, 1999. http://www.cdc.gov/ncidod/dbmd/diseaseinfo/cholera/top.pdf (accessed 1 March 2010). [ Links ]
3. World Health Organization. Cholera: global surveillance summary, 2008. Wkly Epidemiol Rec 2009;84(31):309-324. [ Links ]
4. World Health Organization. Daily cholera update and alerts. 30 July 2009. http://www.who.int/hac/crises/zwe/ sitreps/zimbabwe_cholera_update_30july2009.pdf (accessed 1 September 2009). [ Links ]
5. OCHA. Regional Update No. 10 - Cholera/acute watery diarrhoea outbreaks in southern Africa. 1 June 2009. http://ochaonline.un.org/OchaLinkClick.aspx?link=ocha&docId=1111105 (accessed 2 March 2010).
6. Bhattacharya S, Black R, Bourgeois L, et al. The cholera crisi s in Africa. Science 2009;324:885. [ Links ]
7. World Health Organization. Cholera vaccines: WHO position paper. Wkly Epidemiol Rec 2010;85(13):117-128. [ Links ]
8. World Health Organization. Meeting of the Strategic Advisory Group of Experts on Immunization, October 2009 - conclusions and recommendations. Wkly Epidemiol Rec 2009;84(50):517-532. [ Links ]
9. Zimbabwe Health Cluster. Cholera outbreaks - co-ordinated preparedness and response. Harare, Zimbabwe. November 2008. http://www.who.int/hac/crises/zwe/zimbabwe_cholera_resp_plan_nov08.pdf (accessed 30 September 2009). [ Links ]
10. World Health Organization. Global, national efforts must be urgently intensified to control Zimbabwe cholera outbreak. http://www.who.int/mediacentre/news/releases/2009/cholera_zim_20090130/en/index.html (accessed 28 February 2011). [ Links ]
11. World Health Organization. Cholera in Zimbabwe: Epidemiological Bulletin No. 9 Week 6 (01-07 Feb 2009). http://www.who.int/hac/crises/zwe/zimbabwe_epi_bulletin_9_1_7feb2009.pdf (accessed 10 November 2009). [ Links ]
12. United Nations, Department of Economic and Social Affairs, Population Division (2007). World Population Prospects: The 2006 Revision. CD-ROM edition - extended dataset in Excel and ASCII formats (United Nations publication, Sales No. E.07.XIII.7). [ Links ]
13. Lucas ME, Deen JL, Seidlein L, et al. Effectiveness of mass oral cholera vaccination in Beira, Mozambique. N Engl J Med 2005;352(8):757-767. [ Links ]
14. World Health Organization. Life Tables for WHO Member States. http://www.who.int/healthinfo/statistics/ mortality_life_tables/en/ (accessed 18 March 2010). [ Links ]
15. Murray CJL, Lopez AD, eds. The Global Burden of Disease. Vol. 1 of Global Burden of Disease and Injury Series. Cambridge, Mass: Harvard University Press, 1996. [ Links ]
16. Ali M, Emch M, von Seidlein L, et al. Herd immunity conferred by killed oral cholera vaccines in Bangladesh: A reanalysis. Lancet 2005;366:44-49. [ Links ]
17. Longini IM, Nizam A, Ali M, Yunus M, Shenvi N. Controlling endemic cholera with oral vaccines. PLoS Med 2007;4:e336. [ Links ]
18. Jeuland M, Cook J, Poulos C, Clemens J, Whittington D, DOMI Cholera Economics Study Group. Costeffectiveness of new-generation oral cholera vaccines: A multisite analysis. Value in Health 2009;12(6):899-908. [ Links ]
19. World Health Organization. cMYP database A new approach to planning for immunization - WHO-UNICEF guidelines for developing a comprehensive multi-year plan (cMYP). http://www.who.int/immunization_financing/countries/en/ (accessed 5 March 2010). [ Links ]
20. United Nations OCHA. Zimbabwe - cholera update. Situation report #22 (15 July 2009). http://ochaonline.un.org/cholerasituation/tabid/5147/language/en-us/default.aspx (accessed 15 February 2010) [ Links ]
21. United Nations. Zimbabwe Consolidated Appeal Revision - May 2009. http://ochadms.unog.ch/quickplace/cap/main.nsf/h_Index/Revision_2009_Zimbabwe/$FILE/Revision_2009_Zimbabwe_SCREEN.pdf?OpenElement (accessed 15 February 2010). [ Links ]
22. Mason PR. Emerging problems in infectious diseases: Zimbabwe experiences the worst epidemic of cholera in Africa. J Infect Developing Countries 2009;3(2):148-151. [ Links ]
23. Burki T. Slow progress towards sanitation goal. Lancet 2009;9:531. [ Links ]
24. World Health Organization. Cholera in Zimbabwe Aug08 - Jan09. www.who.int/entity/hac/crises/zwe/zimbabwe_cholera_aug08_jan09.pdf (accessed 30 September 2009). [ Links ]
25. International Vaccine Institute. Oral cholera vaccine: First licensed vaccine developed with Gates Foundation support. http://www.ivi.org/popup/files/ocv_article.pdf (accessed 15 March 2010). [ Links ]
Accepted 12 May 2011.
Conflict of interest. The authors have no competing interests to declare.
Funding statement. This work was supported in part by the Center for Health Decision Science, Harvard School of Public Health. SK is partially supported through the Harvard Global Health Institute (Decision Science Working Group). SG is partially supported by the Bill and Melinda Gates Foundation. The funding source did not have any influence on the design and conduct of the study.
Corresponding author: S-Y Kim (sunyoung.m.kim@gmail.com)














