Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.101 n.9 Pretoria Sep. 2011
ORIGINAL ARTICLES
Should baseline PSA testing be performed in men aged 40 to detect those aged 50 or less who are at risk of aggressive prostate cancer?
C F HeynsI; M FisherII; A LecuonaII; A van der MerweIII
IMB ChB, MMed (Urol), PhD, FCSSA (Urol). Department of Urology, Stellenbosch University and Tygerberg Hospital, Tygerberg
IIMB ChB, MMed (Urol), FC Urol SA. Department of Urology, Stellenbosch University and Tygerberg Hospital, Tygerberg
IIIMB ChB, MRCS, MMed (Urol), FC Urol SA. Department of Urology, Stellenbosch University and Tygerberg Hospital, Tygerberg
ABSTRACT
OBJECTIVE: We aimed to evaluate the presenting features and treatment outcome of prostate cancer in men aged <50 years, in a region where prostate specific antigen (PSA) screening is not readily available and most men present with symptoms.
METHODS: We analysed the data of 1 571 men with prostatic adenocarcinoma treated between January 1997 and December 2008 at our institution, a tertiary level public sector hospital serving a largely indigent population. Statistical analysis was performed using Student's, the Mann-Whitney and Fisher's exact tests where appropriate (p<0.05 accepted as statistically significant).
RESULTS: Of 1 571 men, 47 (3%) were aged <50 years. The group aged <50 years, compared with that aged >50 years, had a significantly greater proportion with poorly differentiated adenocarcinoma (53%), locally advanced (stage T3 - 4) tumours (56%), haematogenous metastases (75%), significantly higher serum PSA at diagnosis (mean 621, median 74 ng/ml) and shorter survival.
CONCLUSIONS: Men aged <50 years presenting with symptoms owing to prostate cancer had significantly higher-risk disease, higher mean PSA, and poorer prognosis than men aged >50 years. To diagnose prostate cancer at a potentially curable stage in men aged <50 years, it is necessary to initiate baseline PSA testing at age 40 and 45 years, and to select high-risk men for PSA surveillance in order to diagnose potentially curable cancer in those with a life expectancy >20 - 25 years.
Although prostate specific antigen (PSA) screening of asymptomatic men aged 50 - 75 years may reduce the prostate cancer mortality by at least 20%, there is concern about the high rate of overtreatment.1 The risk of overtreatment is increased in older men with less aggressive cancer. In men younger than 50 years - especially those with highrisk prostate cancer - early diagnosis and curative treatment are more important than in elderly men, and the risk of overtreatment is less.
Before the advent of widespread PSA testing, men <50 years old comprised 0.8 - 1.1% of patients diagnosed with adenocarcinoma of the prostate in the USA.2 With the widespread use of PSA testing and public awareness of prostate cancer, this figure in the USA is currently 3.7 - 4%.3
Pre-PSA era studies suggested that prostate cancer in younger men is more aggressive, presents at a more advanced stage, responds poorly to radiation or hormonal therapy, and has a worse prognosis than in older men.4 Other studies have suggested that younger men present with more favourable disease and have better survival outcomes than older men.5 Some reports suggested no significant difference in clinical stage at diagnosis or disease recurrence rates between younger and older men.6 Recent studies have shown that younger men in the PSA era have better outcomes than older men.3,5,7
We aimed to evaluate the presenting features and treatment outcome of prostate cancer in men aged <50 years, in a region where PSA screening is not available and most men present with symptoms.
Materials and methods
We analysed the data of 1 571 men diagnosed with adenocarcinoma of the prostate in the period January 1997 to December 2008 at our institution, a tertiary level public sector hospital serving a largely indigent population. The majority presented with symptoms, because PSA testing is not readily available at clinics or hospitals in our referral area. Because many studies suggest that prostate cancer is more common and more aggressive in Blacks, the study data were also analysed for patients' race.
Histological grading was divided into well differentiated (MD Anderson grade 1, Gleason score 2 - 4), moderately differentiated (G2, Gleason 5 - 7) and poorly differentiated (G3, Gleason 8 - 10). Clinical tumour stage on digital rectal examination was categorised as organ-confined (T1 - 2) or locally advanced (T3 - 4). Haematogenous metastases were evaluated with X-rays of the axial skeleton and radionuclide scintigraphy in selected patients, and were categorised as absent (M0) or present (M1).
Statistical analysis was performed using Student's t-test for parametric data, Mann-Whitney test for non-parametric data, and Fisher's exact test for contingency tables. A two-tailed p-value <0.05 was accepted as statistically significant. Ethical approval was provided by the health research ethics committee of the University of Stellenbosch.
Results
Of the 1 571 men, 47 (3%) were aged <50 years. Race was selfassigned as white in 27.3%, coloured in 65.1% , and black in 7.7%. This racial distribution reflects our hospital patients' population demographics. In the group of men <50 years old, the proportion of white men was significantly lower, and the proportion of coloured men was higher than in the age group >50 years (Fig. 1).

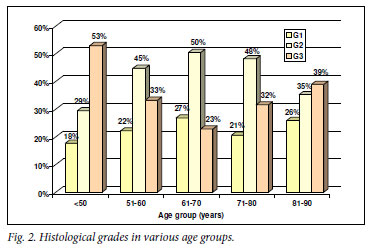
The proportion of high-grade tumors was significantly greater in the age group <50 years (53%) than in any of the other age groups (Fig. 2). The proportion of locally advanced (T3 - 4) tumours was significantly greater in the age group <50 years (56%) than in the age groups 51 - 60 and 61 - 70 years (42%), but smaller than in the age group 81 - 90 years (74%) (Fig. 3). The proportion of men with haematogenous metastases was significantly greater in the group aged <50 years (75%) than the other age groups (Fig. 3).

Mean and median serum PSA at diagnosis was significantly higher in the group aged <50 years than the other groups (Fig. 4). In the group <50 years old, 39% had a serum PSA <20 ng/ml, 22% had a PSA 21 - 99 ng/ml, and 39% had a PSA >100 ng/ml at diagnosis, ranging from 105.0 - 7 000.0 ng/ml. Five men had a PSA >1 000 ng/ml.

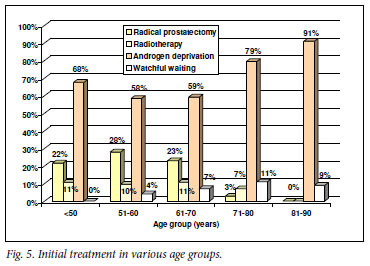
Potentially curative therapy (radical prostatectomy or radiotherapy) was used in a similar proportion of patients in the group aged <50 years as in the groups aged 51 - 70 years (Fig. 5).
Mean and median follow-up (reflecting overall survival) was significantly shorter in the groups aged <50 and 81 - 90 years than in the other groups (Fig. 6).

Discussion
In this study of men with prostate cancer who were diagnosed because of symptomatic presentation and not through PSA screening of asymptomatic men, the proportion aged <50 years (3%) was higher than that reported in the USA during the pre-PSA era (0.8 - 1.1%), and lower than reported in the PSA era (3.7 - 4%). 3
The features of aggressive prostate cancer (high grade, advanced stage, high serum PSA) were significantly more common in men aged <50 years in our study population, which corresponds with studies from the USA in the pre-PSA era,4 but is contrary to studies reporting more favourable disease at presentation and better treatment outcomes in younger men.3,5,7
Changes in PSA levels over time (PSA kinetics) can help to detect potentially fatal prostate cancer. Carter et al. (1992) first reported that a PSA velocity (PSAV) >0.75 ng/ml/year indicated an increased Fig. 1. Race distribution in various age groups. risk for the development of prostate cancer.8 Other studies have shown that a PSAV >2 ng/ml/year, calculated by linear regression, was associated with an increased risk of death from prostate cancer.9
In men with untreated prostate cancer, PSA levels increase initially linearly, followed by an exponential phase.9 Therefore, PSA doubling time (PSADT) calculated using log-linear regression may be a more appropriate measure of cancer growth. A shorter PSADT is associated with a higher risk of death from prostate cancer.10
It has been suggested that a screening interval of 3 years is appropriate for men with a PSA <1 ng/ml, and that it seems safe to offer repeat PSA screening after 2 years for men with a PSA <2 ng/ml, since rapidly growing interval cancers appear to be rare.11 It has been suggested that men with a PSA of 2 - 3 ng/ml may be better served with a shorter screening interval.12
A computer model13 to simulate the natural history of prostate cancer to determine the optimal starting age for PSA testing showed that annual PSA testing from the age 50 years compared with no screening prevented 3.2 deaths, with an additional 10 500 PSA tests and 600 prostate biopsies. PSA testing at ages 40 and 45 years followed by biennial testing beginning at age 50 years prevented 3.3 deaths, with an additional 7 500 PSA tests and 450 prostate biopsies. The authors concluded that screening men at age 40 and 45 years with a 2-year testing interval after age 50 years (or before if the PSA level >2.0 ng/ml), while holding the PSA threshold for a prostate biopsy at 4.0 ng/ml, may be more efficient than annual PSA screening from the ages of 50 - 75 years, because it could prevent an equal number of prostate cancer deaths and reduce the number of PSA tests administered and biopsies performed.13
Men with PSA levels >2.0 ng/ml are more than 12 times as likely to be diagnosed with prostate cancer within the next decade compared with those with PSA levels <1.0 ng/ml.14 These data support the rationale for increased surveillance of young men with normal PSA levels >2.0 ng/ml, while continuing to use 4.0 ng/ml as the cut-off for taking a biopsy.13
In our study of men <50 years who presented because of symptoms and were diagnosed with prostate cancer, 39% had a PSA >100 ng/ ml at diagnosis, ranging from 105.0 -7 000.0 ng/ml. Postulating an exponential rather than linear PSA increase and a PSADT of <12 months in these men presenting with PSA>100 at the age of 50 years, it is clear that to detect cancer at a potentially curable stage (PSA <20 ng/ml) would have required PSA testing at the age of 40, or even earlier.
In a large cohort of men aged <50 years with a PSA <4 ng/ml at the first PSA screening, black and white men with an initial PSA in the 1.5 - 2.4 ng/ml range had a 9.3- and 6.7-fold increase in the risk for prostate cancer, respectively. These data suggest that an initial PSA cutoff of 1.5 ng/ml may be better than a median PSA of 0.7 ng/ml to determine the risk of prostate cancer in men aged <50 years.15
An important problem in prostate cancer treatment was succinctly summarised in the question posed by W F Whitmore: 'If cure is possible, is it necessary? And if it is necessary, is it possible?'16 Many prostate cancers are not aggressive and, even if untreated, will not cause the patient's death. However, some prostate cancers are so aggressive that no combination of therapeutic modalities can effect cure. However, it is clear that early-stage cancer has a greater chance of cure than advanced cancer.16
Although PSA screening of asymptomatic men may reduce prostate cancer mortality, there is a high rate of overtreatment, especially in older men.1 As a public health measure, PSA screening is most probably not cost-effective, especially because prostate cancer mainly affects older men who are not economically productive.17 Nevertheless, from the individual's point of view, it is preferable to diagnose and treat prostate cancer at a potentially curable stage, rather than suffer the extreme pain of skeletal metastases in terminal prostate cancer.
Conclusions
In our referral population, where PSA testing is not readily available and most men present with symptoms, 3% of patients diagnosed were aged <50 years. Of them, a significantly greater proportion had high-grade adenocarcinoma, locally advanced or metastatic disease, serum PSA >100 ng/ml, and shorter overall survival followup compared with those >50 years old. To diagnose prostate cancer at a potentially curable stage in men <50 years old, initial baseline PSA testing must be extended to the age range 40 - 45 years, to select men at increased risk of cancer (PSA >0.7 - 1.5 ng/ml). Annual PSA surveillance should be targeted at these high-risk men, with the aim of diagnosing prostate cancer at a potentially curable stage in men with life expectancy >20 - 25 years. In men at low risk of developing prostate cancer, PSA testing can be deferred to the age of 50 and can be performed at less frequent intervals (3 - 4 times yearly); this may be a more cost-effective way of detecting aggressive prostate cancer at a curable stage.
Acknowledgement. We thank Mrs Mariëtte Pretorius for entering the clinical data on the Urological Oncology database.
References
1. Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med 2009;360:1320-1328. [ Links ]
2. Huben R, Natarajan N, Pontes E, et al. Carcinoma of the prostate in men less than 50 years old. Data from American College of Surgeons' National Survey. Urology 1982;20:585-588. [ Links ]
3. Khan MA, Han M, Partin AW, et al. Long-term cancer control of radical prostatectomy in men younger than 50 years of age: update 2003. Urology 2003;62:86-92. [ Links ]
4. Sandhu DP, Munson KW, Benghiat A, et al. Natural history and prognosis of prostate carcinoma in adolescents and men under 35 years of age. Br J Urol 1992;69:525-529. [ Links ]
5. Carter HB, Epstein JI, Partin AW. Influence of age and prostate-specific antigen on the chance of curable prostate cancer among men with nonpalpable disease. Urology 1999;53:126-130. [ Links ]
6. Smith CV, Bauer JJ, Connelly RR, et al. Prostate cancer in men age 50 years or younger: a review of the Department of Defense Center for Prostate Disease Research multicenter prostate cancer database. J Urol 2000;164:1964-1967. [ Links ]
7. Ruska KM, Partin AW, Epstein JI, et al. Adenocarcinoma of the prostate in men younger than 40 years of age: diagnosis and treatment with emphasis on radical prostatectomy findings. Urology 1999;53:11791183. [ Links ]
8. Carter HB, Morrell CH, Pearson JD, et al. Estimation of prostatic growth using serial prostate-specific antigen measurements in men with and without prostate disease. Cancer Res 1992;52:3323-3328. [ Links ]
9. Teahan SJ, Klotz LH. Current role of prostate-specific antigen kinetics in managing patients with prostate cancer. BJU Int 2006;97:451-455. [ Links ]
10. Sengupta S, Myers RP, Slezak JM, et al. Preoperative prostate specific antigen doubling time and velocity are strong and independent predictors of outcomes following radical prostatectomy. J Urol 2005;174:2191-2196. [ Links ]
11. Carter HB, Epstein JI, Chan DW, et al. Recommended prostate-specific antigen testing intervals for the detection of curable cancer. JAMA 1997;277:1456-1460. [ Links ]
12. Hugosson J, Aus G, Lilja H, et al. Prostate specific antigen based biennial screening is sufficient to detect almost all prostatic cancers while still curable. J Urol 2003;169:1720-1723. [ Links ]
13. Ross KS, Carter HB, Pearson JD, et al. Comparative efficiency of prostate-specific antigen screening strategies for prostate cancer detection. JAMA 2000;284:1399-1405. [ Links ]
14. Gann PH, Hennekens CH, Stampfer MJ. A prospective evaluation of plasma prostate-specific antigen for detection of prostatic cancer. JAMA 1995;273:289-294. [ Links ]
15. Tang P, Sun L, Uhlman MA, et al. Initial prostate specific antigen 1.5 ng/ml or greater in men 50 years old or younger predicts higher prostate cancer risk. J Urol 2010;183:946-951. [ Links ]
16. Heyns CF, Van der Merwe A. Prostate cancer management - helping your patient choose what is best for him. SA Fam Pract 2008;50(5):27-34. [ Links ]
17. Heyns CF, Van der Merwe A. Prostate specific antigen -brief update on its clinical use. SA Fam Pract 2008;50(2):19-24. [ Links ]
Accepted 12 April 2011.
Corresponding author: C F Heyns (cfh2@sun.ac.za)














