Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.101 n.7 Pretoria Jul. 2011
ORIGINAL ARTICLES
APRI: a simple bedside marker for advanced fibrosis that can avoid liver biopsy in patients with NAFLD/NASH
Frederik Cornelis KrugerI; Caroline Rachel DanielsII; Martin KiddIII; Gillaum SwartIV; Karen BrundynIV; Christo van RensburgVI; Maritha KotzeV
IMB ChB, MMed (Int), PhD (Int), Cert Gastro (SA). Gastroenterology Unit, Durbanville Medi-Clinic, Cape Town
IIBA (Cur) (Nursing Science). Gastroenterology Unit, Durbanville Medi-Clinic, Cape Town
IIIPhD (Statistics). Centre for Statistical Consultation, Stellenbosch University
IVMB ChB, MMed (Anat Path). Department of Anatomical Pathology, Faculty of Health Sciences, Stellenbosch University
VPhD (Hum Genet). Department of Anatomical Pathology, Faculty of Health Sciences, Stellenbosch University
VIMB ChB, MMed (Int), PhD (Int). Department of Internal Medicine, Faculty of Health Sciences, Stellenbosch University
ABSTRACT
BACKGROUND: Non-alcoholic steatohepatitis (NASH) can lead to cirrhosis and hepatocellular carcinoma. The NASH fibrosis score (NFS) has proven to be a reliable, non-invasive marker for prediction of advanced fibrosis. Aspartate aminotransferase-toplatelet ratio index (APRI) is a simpler calculation than NFS, but has never been studied in patients with non-alcoholic fatty liver disease (NAFLD).
AIM: To validate APRI as a non-invasive marker of liver fibrosis in subjects with NAFLD to be used in clinical practice.
DESIGN/METHODS: The cohort consisted of 111 patients with histological diagnoses of NAFLD. The biopsy samples were staged and graded according to the NASH clinical research network (CRN) criteria. These were grouped into fatty liver disease (FLD), NASH, no/mild fibrosis, and advanced fibrosis. The sensitivity and specificity of APRI were compared with NFS and aspartate aminotransferase-to-alanine aminotransferase (AST/ALT) ratio.
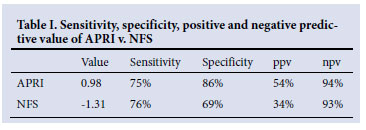
RESULTS: The APRI was significantly higher in the advanced fibrosis group. The area under receiver operating characteristic (ROC) curve for APRI was 0.85 with an optimal cut-off of 0.98, giving a sensitivity of 75% and a specificity of 86%. The NFS was significantly lower in the advanced fibrosis group. The ROC for NFS gave an area under curve (AUC) of 0.77 and a cut-off value of -1.3 with a sensitivity of 76% and specificity of 69%. The positive predictive value for APRI was 54% as opposed to 34% for NFS. The negative predictive value was 93% for APRI and 94% for NFS.
CONCLUSION: APRI compared favourably to NFS and was superior to AST/ALT for the prediction of advanced fibrosis. We therefore propose the use of APRI in a new algorithm for the detection of advanced fibrosis.
Non-alcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease in the world,1-3 affecting about a third of the USA's population.4 Non-alcoholic steatohepatitis (NASH) is the non-benign form of NAFLD potentially leading to liver cirrhosis but also to hepatocellular carcinoma.5,6 There is no difference in the management of patients with NASH with minimal forms of fibrosis and without fibrosis. However, patients with NASH and advanced forms of fibrosis must be identified as they will require more intensive management. The assessment of patients with NAFLD/NASH for advanced disease by liver biopsy is regarded as the gold standard.4 However, a liver biopsy sampling error can result in substantial misdiagnosis and staging inaccuracies.7 The main concern regarding liver biopsies is the lack of resources, largely because of the large number of subjects affected by NAFLD. Secondly, approximately 60 -90% of NAFLD-affected subjects have a benign form of the disease not requiring biopsy.8-10 Furthermore, liver biopsy is invasive with potential complications.11
Aminotransferase levels do not correlate with underlying biological activity and can even be normal in advanced disease.12-14 An aspartate aminotransferase-to-alanine aminotransferase (AST/ALT) ratio of more than 1 may suggest advanced fibrosis or cirrhosis.15 Numerous test panels have been developed for non-invasive tests to diagnose advanced liver disease,16 only 4 of which have been evaluated in NAFLD.4 The group that investigated the BAAT (BMI, ALT, age, triglycerides) score replaced this test by the FibroTest.17 The FibroTest combines 5 biochemical markers, namely β2-macroglobulin, apolipoprotein A1, haptoglobulin, total bilirubin and gamma-glutamyl transpeptidase (GGT). Age and sex, together with the aforementioned markers, are entered into a computer programme using an undisclosed formula. The area under curve (AUC) for the FibroTest as predictive of advanced fibrosis is 0.87. Unfortunately, the FibroTest is expensive and not widely available. The European Liver Fibrosis (ELF) Study Group examined a panel of extracellular matrix (ECM)-related components from which an algorithm was developed with an AUC for severe fibrosis of 0.87.18 This panel is also not readily available and will be expensive, especially in developing countries.
The NASH fibrosis score (NFS) is an algorithm of 6 readily available laboratory and clinical variables including age, hyperglycaemia, BMI, platelet count, albumin, and AST/ALT ratio.19 By applying this model, 75% of 733 patients in this study avoided liver biopsy. Guha et al.20 determined that the addition of established simple markers to the ELF panel augmented the diagnostic performance and that liver biopsy could be avoided in 88% of cases. Wai et al. validated the aspartate aminotransferase-to-platelet ratio index (APRI) score in patients with hepatitis C.21 APRI is a simple calculation of two laboratory variables, namely AST and platelets. This score can easily be used at the bedside or in an outpatient setting. APRI has not been validated for use in NAFLD/NASH and has not been compared with other non-invasive markers for advanced fibrosis in NAFLD/NASH.
We therefore aimed to validate APRI as a non-invasive marker of advanced fibrosis in subjects with NAFLD. Furthermore, by proving superior sensitivity and specificity of APRI compared with AST/ ALT ratio and comparable sensitivity and specificity to NFS, APRI can be used as part of a proposed simple, user-friendly and reliable algorithm to predict advanced fibrosis in subjects with NAFLD, thereby avoiding liver biopsies for patients with no or minimal fibrosis.
Methods
Patients
The study included 111 patients with histologically confirmed NAFLD recruited from 3 sites in the Western Cape province of South Africa, i.e. Tygerberg Academic Hospital, and Louis Leipoldt and Durbanville Medi-Clinics. The study was approved by the regulatory body of Stellenbosch University. Patient age, sex, body mass index (BMI), history of diabetes and detailed alcohol consumption history were recorded. Patients who consumed more than 140 g of alcohol per week were excluded. Other liver diseases were also excluded. Clinical and laboratory data were collected either before or on the day of the liver biopsy. BMI was calculated using the formula: weight in kg/height in metres2.
Biochemistry
Laboratory evaluation included full liver function tests, full blood count, fasting glucose and fasting insulin. Insulin resistance was determined by using the homoeostasis model assessment (HOMA) formula = insulin x glucose/22.5. APRI was calculated by using the formula: (AST/upper limit of normal x 100)/platelet count. NFS by Angulo et al.: 1.675 + 0.037 x age (years) + 0.094 x BMI (kg/m2) + 1.13 x IFG/diabetes (yes=1, no=0) + 0.99 x AST/ALT ratio - 0.013 x platelets (x 109/l) - 0.66 x albumin (g/dl).
Histology
The same two pathologists reported on each sample that were specially stained to exclude iron and copper overload. These were staged and graded according to the NASH National Institute of Health Chronic Research Network criteria. The samples were classified into four histologically defined groups, i.e. fatty liver disease not fulfilling the criteria for NASH (FLD), NASH, no or mild fibrosis (stage 1 and 2) and advanced fibrosis (stage 3 and 4). ALT, AST/ALT ratio, APRI and NFS were performed for each group, and compared for predictiveness of advanced NAFLD.
Statistical analysis
One-way ANOVA was used to compare average measurements between different groups of patients. Possible deviations from the assumptions were checked and highlighted in cases where they caused a problem. The non-parametric Mann-Whitney U-test was then used. Receiver operating characteristic (ROC) analysis was used to determine optimal cut-off points for diagnosis. AUC, sensitivity, specificity, positive predictive value (ppv) and negative predictive value (npv) were reported.
Results
Patient demographics
Our subjects were ethnically classified as: 69% coloured, 25% white, 5% black and 1% Indian; 73% were female. The mean age of the cohort was 52 years (confidence interval (CI) 50 - 54 yrs); mean BMI was 35 (CI 34 - 36); and 43% were type II diabetics. The mean homeostasis model of assessment - insulin resistance (HOMA-IR) of the non-diabetic patients was 7 (CI 4 - 9), 41% of the patients had NASH, and 17% had advanced fibrosis. None had decompensated liver disease.
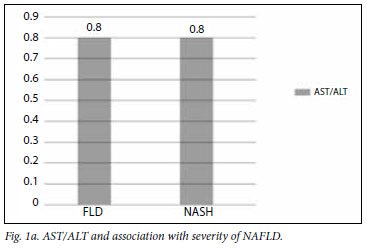
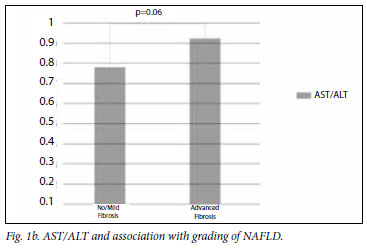
The mean AST/ALT ratio for the different groups is illustrated in Figs 1a and 1b, showing a trend towards a higher value for patients with advanced fibrosis.
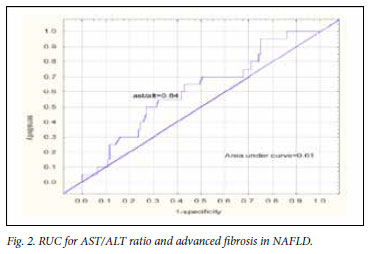
The AUC is illustrated in Fig. 2. The AUC was 0.61 with an AST/ ALT ratio of 0.8, having a sensitivity of 58% and specificity of 62%.
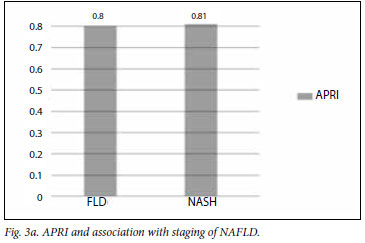
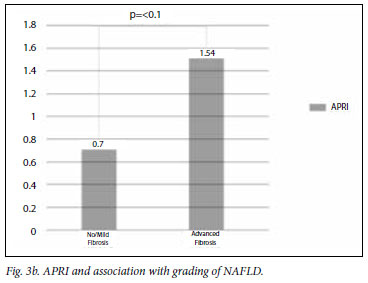
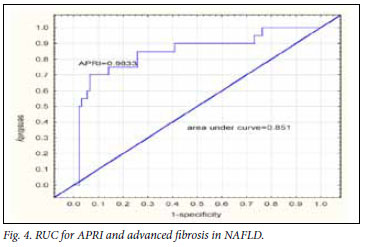
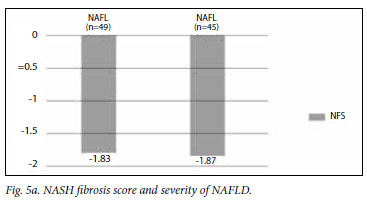
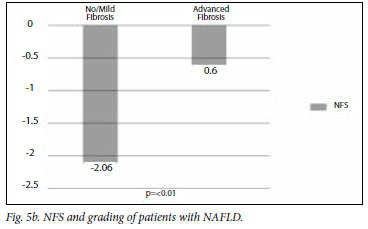
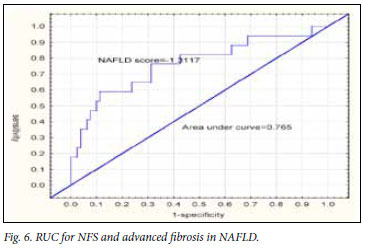
The mean APRI for the groups is illustrated in Figs 3a and 3b. The APRI was significantly higher in the advanced fibrosis group. The AUC for APRI is illustrated in Fig. 4. The AUC for APRI was 0.85 with a cut-off of 0.98, giving a sensitivity of 75% and a specificity of 86%. The mean NFS for the different groups is illustrated in Figs 5a and 5b, showing that the NFS was significantly lower in the advanced fibrosis group. The AUC for NFS is illustrated in Fig. 6. The AUC for NFS was 0.77 given at a cut-off of -1.31. The sensitivity and specificity for NFS was 76% and 69% respectively. The sensitivity, specificity, and positive and negative predictive values for APRI and NFS are compared in Table I. The positive predictive value for APRI was 54%, as opposed to 34% for NFS. The negative predictive value was 93% for APRI and 94% for NFS.
Discussion
Liver biopsy is regarded as the gold standard in the assessment of patients with NAFLD/NASH for advanced disease.22 However, liver biopsy is an invasive procedure with potential complications, and sampling error can result in substantial misdiagnosis and staging inaccuracies. The major concern regarding liver biopsies is the lack of resources mainly owing to the large number of subjects affected by NAFLD. Approximately 60 -90% of NAFLD-affected subjects have a benign form of the disease not requiring biopsy. The management of patients with NASH and no and minimal fibrosis does not differ, whereas patients with advanced forms of fibrosis must be identified for more intensive management.23 Improved methods are therefore required to identify patients at increased risk of severe liver disease without needing to perform a liver biopsy on all patients. Numerous test panels have been developed for non-invasive tests to diagnose advanced liver disease, of which only 4 have been evaluated in NAFLD.4 The group that investigated the BAAT score replaced this test by the FibroTest, which unfortunately is expensive and not widely available. The European Liver Fibrosis Study Group panel of ECMrelated components from which an algorithm was developed, is also not readily available and will be expensive, especially in developing countries. The NFS is an algorithm of 6 readily available laboratory and clinical variables including age, hyperglycaemia, BMI, platelet count, albumin, and AST/ALT ratio.19 By applying this model, almost 75% of the 733 patients in this study could have avoided liver biopsy. Guha et al.20 determined that adding established simple markers to the ELF panel augmented the diagnostic performance.20 Wai et al.21 validated the APRI score in patients with hepatitis C. APRI is a simple calculation of 2 laboratory variables -AST and platelets; this score can easily be used at the bedside or in an outpatient setting.
We attempted to validate APRI as a non-invasive marker of advanced fibrosis in subjects with NAFLD. By proving superior sensitivity and specificity of APRI compared with AST/ALT ratio and comparable sensitivity and specificity with NFS, the use of APRI is proposed as part of a simple, user-friendly and reliable algorithm to predict advanced fibrosis in subjects with NAFLD and thereby avoiding liver biopsies for patients with no or minimal fibrosis. Our study confirmed that ALT could neither differentiate between the stage of disease nor the grade of fibrosis. An AST/ALT ratio >0.8 is an indicator of advanced disease. There was a strong tendency in the South African study towards subjects with advanced fibrosis having a higher ratio. However, the ROC curve for AST/ALT ratio and advanced fibrosis was only 0.61, indicating that the positive and negative predictive values were too low to make it a useful tool. APRI is a simple and inexpensive calculation making use of the AST value and platelet count. The formula has been validated in patients with hepatitis C but not in patients with NAFLD. Our study showed that the APRI for South African patients with advanced fibrosis differed significantly from that in patients with less severe disease. The ROC curve for an APRI of 0.98 and detection of advanced fibrosis was 0.85, with positive and negative predictive values of 54% and 94% respectively.
Based on these findings, APRI is statistically superior to the AST/ ALT ratio for predicting advanced fibrosis, and has been validated for use in patients with NAFLD for the first time. The NFS was validated by Angulo et al.19 for use in patients with NAFLD. Our results were similar by showing that subjects with advanced fibrosis had a NFS significantly different from that in subjects without advanced fibrosis. The AUC curve for the NFS of -1.31 and prediction of advanced fibrosis was 0.765. The positive and negative predictive values were 34% and 93% respectively.
This study confirmed that APRI is useful for detecting advanced fibrosis in subjects with NAFLD, and compares favourably with NFS to predict advanced fibrosis. However, APRI is easier to use than NFS, is inexpensive and can be used in an outpatient setting and at the bedside. The positive predictive values of these 2 tests were low. According to Guha et al.,20 the addition of the ELF panel to the NFS increased the positive predictive value for advanced fibrosis.
References
1. Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 2004;40:1387-1395. [ Links ]
2. Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: The Dionysos nutrition and liver study. Hepatology 2005;42:42-52. [ Links ]
3. Fan JG, Zhu J, Li XJ, et al. Prevalence of and risk factors for fatty liver in the general population of Shanghai, China. J Hepatol 2005;43:508-514. [ Links ]
4. Wieckowska A, McCullough AJ, Feldstein AE. Noninvasive diagnosis and monitoring of nonalcoholic steatohepatitis: Present and future. Hepatology 2007;46(2):582-589. [ Links ]
5. Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology 1999;116:1413-1419. [ Links ]
6. Bugianessi E, Leone N, Vanni E, et al. Expanding the natural history of non-alcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology 2002;123:134-140. [ Links ]
7. Ratziu V, Charlotte F, Heurtier A, et al. Sampling variability of liver biopsy in non-alcoholic fatty liver disease. Gastroenterology 2005;128:1898-1906. [ Links ]
8. Adams LA, Lymp JF, St Sauver J, et al. The natural history of non-alcoholic fatty liver disease: a population-based cohort study. Gastroenterology 2005;129:113-121. [ Links ]
9. Ekstedt M, Franzen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 2006;44:865-873. [ Links ]
10. Kruger FC, Daniels C, Swart G, Van Rensburg CJ, Kidd M, Hall P. Correlation of body mass index (BMI) with the different types of nonalcoholic fatty liver disease (NAFLD). S Afr Med J 2004;8:682 (abstract). [ Links ]
11. McGill DB, Rakela J, Zinmeister AR, et al. A 21 yr experience with major hemorrhage after percutaneous liver biopsy. Gastroenterology 1990;99:1396. [ Links ]
12. Mofrad P, Contos MJ, Hague M, et al. Clinical and histological spectrum of non-alcoholic fatty liver disease associated with normal ALT values. Hepatology 2003;37:1286-1292. [ Links ]
13. Ruhl CE, Everhart JE. Determinants of the association of overweight with elevated serum alanine aminotransferase activity in the United States. Gastroenterology 2003;124:71-79. [ Links ]
14. Garcia-Monson C, Martin-Perez E, Iacono OL, et al. Characterization of pathogenic and prognostic factors of non-alcoholic steatohepatitis associated with obesity. J Hepatol 2003;33:716-724. [ Links ]
15. Dixon JB, Bhathal PS, O'Brien PE. Nonalcoholic fatty liver disease: predictors of non-alcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology 2001;121:91-100. [ Links ]
16. Rockey DC, Bissel DM. Noninvasive measures of liver fibrosis. Hepatology 2006;43:S113-S120. [ Links ]
17. Ratzui V, Massard J, Charlotte F, et al. Diagnostic value of biochemical markers(Fibro Test- Fibro SURE) for the prediction of liver fibrosis in patients with nonalcoholic fatty liver disease. BMC Gastroenterol 2006;6:6. [ Links ]
18. Rosenberg WM, Voelker M, Thiel R, et al. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology 2004;127:1704-1713. [ Links ]
19. Angulo P, Hui, JM, Marchesini G, et al. The NAFLD fibrosis score: a non-invasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007;45:846-854. [ Links ]
20. Guha IN, Parkers J, Roderick P, et al. Non-invasive markers of fibrosis in non-alcoholic fatty liver disease: Validating the European liver fibrosis panel and exploring simple markers. Hepatology 2008;47:455-460. [ Links ]
21. Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003;38:518-526. [ Links ]
22. Bianchi L. Liver biopsy in elevated liver functions tests? An old question revisited. J Hepatol 2001;35:290-294. [ Links ]
23. Adams LA, Sanderson SO, Lindor KD, et al. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol 2005;42:132-138. [ Links ]
Accepted 18 November 2010.
Corresponding author: F Kruger (ckruger@gastrosa.com)














