Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.101 n.6 Pretoria Jun. 2011
ORIGINAL ARTICLES
Frequency and clinical genetics of familial dilated cardiomyopathy in Cape Town: implications for the evaluation of patients with unexplained cardiomyopathy
Ntobeko B A NtusiI; Ambroise WonkamV; Gasnat ShaboodienII; Motasim BadriIII; Bongani M MayosiIV
IMB ChB, FCP (SA). Cardiac Clinic and Cardiovascular Genetics Laboratory, Hatter Institute for Cardiovascular Research in Africa, Department of Medicine, Groote Schuur Hospital and University of Cape Town
IIPhD. Cardiac Clinic and Cardiovascular Genetics Laboratory, Hatter Institute for Cardiovascular Research in Africa, Department of Medicine, Groote Schuur Hospital and University of Cape Town
IIIPhD (Present address: College of Medicine, King Saud Bin Abdelaziz University of Medical Sciences, Riyadh, Kingdom of Saudi Arabia). Cardiac Clinic and Cardiovascular Genetics Laboratory, Hatter Institute for Cardiovascular Research in Africa, Department of Medicine, Groote Schuur Hospital and University of Cape Town
IVDPhil, FCP (SA). Cardiac Clinic and Cardiovascular Genetics Laboratory, Hatter Institute for Cardiovascular Research in Africa, Department of Medicine, Groote Schuur Hospital and University of Cape Town
VMD. Division of Human Genetics, Departments of Clinical Laboratory Sciences and Medicine, University of Cape Town
ABSTRACT
BACKGROUND: Studies from Europe and North America suggest that 20 - 50% of patients with dilated cardiomyopathy (DCM) may have familial disease. There is little information on the frequency and clinical genetics of familial DCM in Africa.
PURPOSE: To determine the frequency and probable mode of inheritance of familial DCM in patients referred for investigation of the cause of DCM at a tertiary centre in Cape Town.
METHODS: We conducted a retrospective analysis of consecutive patients diagnosed with DCM between 1 February 1996 and 31 December 2009 to determine the frequency of familial disease.
RESULTS: Of 109 unrelated patients with DCM, 29 (26.6%) had familial disease. Their mean age of onset of cardiomyopathy (28.01 (standard deviation (SD) 15.33) years) was significantly younger than that for non-familial cases (39.1 (SD 12.6) years) (p=0.001). Male predominance (N=21, 72.4%) and racial distribution (15 (48.3%) coloured patients, 10 (34.5%) black Africans, 4 (13.8%) white individuals, and 1 (3.4%) of Indian descent) of familial DCM probands were similar to the non-familial cases. Of the 29 patients with familial DCM, 2 (7%) had at least one relative diagnosed with peripartum cardiomyopathy. Pedigree analysis of the 29 families was consistent with autosomal dominant inheritance in 72.4%, autosomal recessive inheritance in 17.2% and X-linked recessive inheritance in 10.4%.
CONCLUSIONS: Familial DCM affects at least a quarter of African patients with DCM, presents at a young age, is associated with peripartum cardiomyopathy, and follows an autosomal dominant pattern of inheritance in the majority of families. Family screening for familial DCM is indicated in all cases of unexplained DCM, including patients with peripartum cardiomyopathy.
Dilated cardiomyopathy (DCM) is defined by the presence of left ventricular systolic dysfunction and dilatation in the absence of abnormal loading conditions (e.g hypertension or valve disease) or coronary artery disease sufficient to cause global systolic impairment.1 Right ventricular dilation and dysfunction may be present, but are not necessary for the diagnosis. DCM is an important cause of heart failure, and a common indication for heart transplantation.2 The clinical and morphological diversity of DCM reflects the broad spectrum of distinct underlying molecular and environmental causes. The prevalence of DCM is estimated to be 36.5/100 000 in a US population.3 There are no population-based studies of the epidemiology of DCM in sub-Saharan Africa.4 Most cases of DCM are thought to be sporadic or acquired.5 However, in many cases the disease is inherited and is termed familial DCM, which may account for up to 20 -50% of DCM in Western populations.5-8
Familial DCM is principally caused by genetic mutations in genes that encode cytoskeletal, nuclear and sarcomeric proteins in the cardiac myocyte.9 In addition, modifying genes, lifestyle and additional factors influence onset of disease, disease progression and prognosis.10 Pedigree analysis in familial DCM is most consistent with autosomal dominant (AD) inheritance with variable penetrance.11
We know of no information on the frequency and clinical genetics of familial DCM in Africa.12 Our aims were: (i) to describe the frequency of familial DCM in patients who were diagnosed with DCM and followed up in a cardiomyopathy clinic at the Cardiac Clinic, Groote Schuur Hospital (GSH), Cape Town, from 1 February 1996 to 31 December 2009; (ii) to determine, through pedigree analysis, the likely modes of inheritance of familial DCM in this cohort; and (iii) to address the implications of the findings for the clinical evaluation of patients with unexplained DCM.
Methods
Study design
This was a retrospective hospital-based study of the frequency and clinical genetics of familial DCM in a cohort of DCM patients referred to the cardiomyopathy clinic in the GSH Cardiac Clinic. The frequency of familial DCM was based on a detailed family history in all individuals and family screening of first-degree relatives of probands with a positive history for confirmation of familial disease. Familial DCM was defined as the presence of DCM in at least one first-degree relative of the proband and/or sudden unexplained death under the age of 35 years in a first-degree relative.13
Study population
We reviewed the medical records of all patients evaluated for a cause of cardiomyopathy from 1 February 1996 to 31 December 2009. These patients were seen in a dedicated cardiomyopathy clinic, where one of the authors (BMM) has a special interest in disorders of heart muscle, and do not reflect the total experience of the GSH Cardiac Clinic. Patients included were those with DCM, as evidenced by clinical signs and symptoms of heart failure associated with left ventricular dilatation and a left ventricular ejection fraction less than 50% on echocardiography or cardiac catheterisation. Patients with any of the following conditions were excluded: rheumatic or other intrinsic valvular heart disease, coronary artery disease, pericardial disease, chronic hypertension, congenital heart disease, and any other systemic disease with cardiovascular sequelae such as diabetes, drug-induced cardiomyopathy, haemochromatosis, neuromuscular disease, infantile endocardial fibro-elastosis, endomyocardial fibrosis, amyloidosis, or myocarditis documented on endomyocardial biopsy or associated with proven viral infection. Patients who drank alcohol were included, since such persons may have a genetic predisposition to DCM and their exclusion could spuriously inflate the proportion of patients with familial DCM because a diagnosis of alcohol-induced cardiomyopathy might be made more readily in the absence of a family history of DCM. For similar reasons, patients with peripartum cardiomyopathy were included. However, patients with hypertrophic, restrictive and tachycardia-related cardiomyopathies were excluded.
Clinical genetics
A two-to five-generation family pedigree was constructed for every patient with DCM. If a subject with DCM had any firstdegree relative who was also affected with DCM, those firstdegree relatives were invited for screening for DCM by history, physical examination, electrocardiogram (ECG), echocardiography and cardiac catheterisation where indicated. For deceased relatives, medical records were reviewed when available. Relatives with other cardiac disorders that could account for left ventricular dysfunction were excluded. Pedigree analysis was performed for all affected families to ascertain the probable pattern of inheritance of familial DCM. Pedigrees were constructed using the Cyrillic 2.1 software programme (http://www.cyrillicsoftware.com/).
Statistical analysis
Results of quantitative variables are given as mean (standard deviation (SD)). Categorical variables are represented as number and percentage. Pearson's chi-square or Fisher's exact test was used to compare the relative frequency of characteristics between individuals. All p-values are two-sided, and p-values <0.05 are considered to indicate statistical significance.
Ethical considerations
The study was approved by the University of Cape Town Faculty of Health Sciences Human Research Ethics Committee (REC Ref. No. 197/96). Participants gave written informed consent. All eligible patients were asked for permission to invite their first-degree relatives (parents, siblings and children) to participate.
Results
Frequency of familial DCM
Of 109 unrelated cases with DCM studied, 29 (26.6%) had familial DCM. They were derived from all the populations of Cape Town and reflected the referral base of GSH, with 15 (48.3%) coloured/ of mixed ancestry, 10 (34.5%) black African, 4 (13.8%) white, and 1 (3.4%) of Indian descent. A total of 40 individuals with familial DCM were finally identified through family screening of the 29 original unrelated patients.
Characteristics of patients with familial DCM
The 29 unrelated cases with familial DCM had a mean age at diagnosis of 28.01 (SD 15.3) years, with a preponderance of males (N=21, 72.4%); their characteristics were comparable to the total of 40 individuals with familial DCM from the 29 families studied (i.e. 72.5% of them were male, with a mean age at the time of diagnosis of familial DCM of 25.58 (SD 15.1) years). The age of the individuals with familial DCM was significantly lower than that of patients with non-familial DCM, who had a mean age of 39.1 (SD 12.6) years at the time of diagnosis (p=0.001) (Table I).
There was no difference in degree of effort intolerance measured by New York Heart Association functional class at presentation between the index cases with familial and non-familial DCM (Table I). The patients with familial and non-familial DCM also had similar heart rates and mean systolic blood pressures at presentation (i.e. 95.6 (SD 23.3) v. 94.9 (SD 19.5) beats/min (p=0.496) and 100.1 (SD 14.9) v. 101.8 (SD 18.1) mmHg (p=0.397), respectively). While the frequency of ECG left bundle-branch block was lower in the familial cases (17.2%) compared with the non-familial cases (36.3%), the difference was not statistically significant (p=0.123). However, patients with familial DCM had significantly less ventricular dilation than the nonfamilial cases, the left ventricular end-diastolic dimension (LVEDD) being 6.2 (SD 1.1) cm in the familial group versus 6.8 (SD 1.4) cm in the non-familial group (p=0.001) (Table I).
Clinical genetics of familial DCM
Table II summarises the pedigree analysis of the families with DCM that were studied. Pedigree analysis of the 29 families was consistent with AD inheritance in 21 families (72.4%). An autosomal recessive (AR) inheritance pattern was observed in 5 families (17.2%). X-linked recessive (X-LR) inheritance was seen in 3 (10.4%) of the familial DCM families. The examples of family pedigrees with AD, AR, and X-LR inheritance are depicted in Fig. 1, and the full list of pedigrees is available online at http://www.medicine.uct.ac.za/Supplementary%20File%20-%20Figure%202.DOC
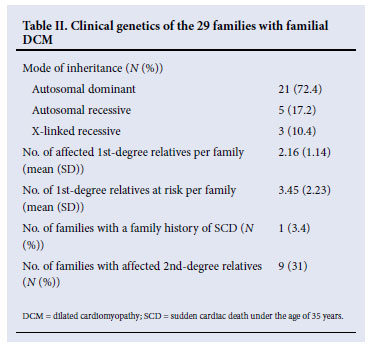

The number of affected first-degree relatives per family was 2.16 (SD 1.14). In these 29 families with DCM, the number of first-degree relatives at risk was 3.45 (SD 2.23) per family. One family (3.4%) had a clear history of a member with sudden cardiac death before the age of 35 years. Of the 29 families included in the analysis, 9 (31.0%) had second-degree relatives who were affected.
It is noteworthy that 2 of the 29 unrelated patients (7%) with familial DCM had at least one relative diagnosed with peripartum cardiomyopathy, confirming that some cases of peripartum cardiomyopathy are part of the spectrum of familial DCM.
Discussion
The first familial cases of cardiomyopathy were reported in 1949;14 subsequently there have been many publications on familial DCM. This study extends the observations made in Western populations to Africa.15 We show that familial DCM occurs in 26.6% of cases of DCM and is a disease of young males with an AD pattern of inheritance in the majority of families. We also confirm that some cases of peripartum cardiomyopathy, which occurred in the context of familial DCM in 2 families, are part of the spectrum of familial DCM.16
Our finding of familial disease in a quarter of DCM patients is in line with the findings of others, who have found inherited disease to constitute 20 - 50% of DCM.5-8,17,18 A prospective echocardiographic study that screened first-degree relatives of patients with DCM estimated the rate of familial DCM to be 20.3%,6 as 315 relatives of 59 index patients with DCM underwent screening with echocardiography, including coronary angiography for those older than 40 years in order to exclude coronary artery disease. DCM was found in 18 relatives (20% of index patients), whereas only 5% had been suspected of having familial disease on the basis of family history alone. In another prospective study of 56 probands with DCM, the definite familial DCM rate was estimated at 25%, where the diagnosis of familial disease was based on first-degree relatives with a diagnosis of DCM on cardiac catheterisation or autopsy, or a first-degree relative with both an echocardiographic LVEDD greater than two SD above the mean and a left ventricular ejection fraction (LVEF) below 50%.19 When left ventricular enlargement was taken as a clinical indicator of DCM, two other studies found the frequency of familial DCM to range from 35%20 to 48%.21
Over 70% of our patients with familial DCM were male. This male preponderance in DCM has been described previously.22,23 In hospital-based studies in sub-Saharan Africa, DCM occurs twice as commonly in men as in women.24 A study of 637 DCM patients, including 130 patients with familial DCM, found that male patients constitute approximately 70% of both non-familial and familial DCM cohorts studied.25
The finding of a younger age of onset of familial DCM compared with non-familial disease is also consistent with other studies and compatible with a genetic cause.25 An earlier systematic study of familial DCM also found it to occur at a younger age (mean age of onset 32 years) compared with patients with idiopathic DCM.26 Another study comparing familial and non-familial DCM found that only a younger age of onset was predictive of familial disease; no other clinical or morphological features were useful in distinguishing the two entities from each other.27 Likewise, Grünig et al. found that 156 of 445 DCM patients confirmed to have familial DCM were much younger than those without familial disease.20 Early-onset cardiovascular disease is likely to be influenced by genetic factors more than late-onset disease.28
We found that at least 70% of familial DCM families demonstrated an AD pattern of inheritance, an observation supported by many previous publications. Familial DCM has been reported most commonly with AD inheritance in up to 90% of cases.8,27,29 The genetic and clinical heterogeneity observed is in keeping with causation by multiple genes, with gene-environment interactions altering expression of disease.27 To date, over 25 mutations have been described in autosomal genes that cause familial DCM.8,29 Some of the commonly mutated genes causing the AD type of familial DCM include lamin A/C, betamyosin heavy chain, alphamyosin heavy chain, actin, alpha-actinin-2, metavinculin, desmin, sarcoglycan, troponin T, alpha-tropomyosin, titin and phospholamban.8 There are also genetic polymorphisms that are associated with an increased risk of developing DCM in some populations.30,31
An AR pattern of inheritance may account for up to 10% of familial DCM, and is commoner in certain ethnic groups and in cases of infantile DCM.32 Unlike many other studies, we found a slightly higher rate of AR inheritance, 17.2%. A mutation in cardiac troponin I has been shown to cause AR DCM in one family.33
X-linked familial DCM has been reported in 5 -10% of cases.23 X-linked types of familial DCM usually result from mutations in the dystrophin gene, which are commonly associated with skeletal muscle weakness and elevated levels of creatinine kinase.8,34,35 In some cases, DCM has been the only presenting feature of patients with Becker muscular dystrophy or in female carriers.36 Furthermore, Becker muscular dystrophy and DCM have been seen in the same family, suggesting that it may be difficult to draw conclusions about phenotype from genotype.37,38 The determination of inheritance patterns by clinical genetic analysis of familial DCM is not straightforward. Use of family history and construction of a three- or four-generation family pedigree may not be sensitive enough when used alone for screening. Often, the phenotypes of affected individuals are not sufficiently specific for familial DCM.13 Furthermore, familial DCM demonstrates incomplete penetrance, age-dependent disease expression and variable expression.39 Among individuals carrying the same gene mutation, there may be wide variability in phenotypic effects and disease severity both within and between families. Within the same family, the phenotype may range from subtle or no symptoms to development of arrhythmia, heart failure, sudden cardiac death, stroke or need for cardiac transplantation.8
Our study observations have major implications for clinical practice. Inherited forms of cardiomyopathy are frequently responsible for heart failure that is otherwise unexplained and often labelled as idiopathic cardiomyopathy before a complete evaluation is conducted. Evaluation of the patient with unexplained cardiomyopathy should include a detailed family history, assessment for the presence of syndromic features, family screening and clinical genetic analysis of first-degree relatives, and consideration of molecular genetic testing. The past 10 years have seen remarkable advances in molecular genetics.15 Improved technology has lowered costs and clinical use of molecular genetic testing is rapidly expanding in South Africa and other parts of the world.40 Genetic counselling about the potential risks and benefits of genetic testing is essential in the clinical care of individuals and families with inherited heart disease. However, the likelihood of finding a responsible gene mutation varies among the different types of inherited cardiomyopathy. In both hypertrophic and right ventricular forms of cardiomyopathy there is a relatively high likelihood of finding a responsible gene mutation when molecular genetic testing is properly applied.15,40 In contrast, the identification of pathogenic mutations in familial DCM is more challenging because of extreme genetic heterogeneity. Nevertheless, the clinical screening of family members at risk for an inherited form of cardiomyopathy leads to earlier identification, earlier treatment, and improved outcomes with or without molecular genetic testing.15,25
This study has the limitations of a retrospective design. Because it was conducted in a tertiary referral centre for inherited cardiomyopathies there may be a referral bias, which may overestimate the true frequency of familial DCM. On the other hand, the number of familial DCM cases in our cohort may have been underestimated for several reasons. First, a varying spectrum of cardiac abnormalities may be present in asymptomatic relatives of patients with familial DCM. Hence, familial and sporadic types of the disease are not easily distinguishable by family history and clinical screening of first-degree relatives in the absence of a 'gold standard' for diagnosis. Second, our diagnosis of familial DCM was based on family history, and screening of first-degree relatives of individuals with a positive family history to confirm the presence of familial DCM. It is therefore likely that we report the minimum frequency of familial DCM, and that the screening of the individuals without a family history is likely to yield a higher prevalence of familial disease.21
Conclusion
We have shown that familial DCM is common in African patients with unexplained DCM, occurring in a quarter of DCM patients studied. We found that familial DCM has a predilection for males and an early age of onset, with the mean age at diagnosis being 28 years. Over 70% of the affected families demonstrated an AD pattern of inheritance. These findings have major implications for the clinical evaluation of patients with unexplained cardiomyopathy (including those with peripartum cardiomyopathy), in whom family screening of first-degree relatives for cardiomyopathy is indicated.
We are grateful to the patients and families who participated in this study. We also acknowledge the expert assistance of Ms Lebogang Montewa, Ms Stella du Toit, Mr Simpiwe Nkepu, Ms Carolina Lemmer, and Sisters Maitele Tshifularo, Unita September and Veronica Francis in the execution of this study.
We have no conflicts of interest to declare.
The authors are funded in part by research grants from the University of Cape Town, the Medical Research Council of South Africa, the Discovery Foundation, the National Research Foundation of South Africa, and the Lily & Ernst Hausmann Research Trust.
References
1. Elliott P, Andersson B, Arbustini E, et al. Classification of the cardiomyopathies: a position statement from the European Society of Cardiology working group on myocardial and pericardial diseases. Eur Heart J 2008;29:270-276. [ Links ]
2. Davies MJ. The cardiomyopathies: An overview. Heart 2000;83:469-474. [ Links ]
3. Codd MB, Sugrue DD, Gersh BJ, Melton LJ III. Epidemiology of idiopathic dilated cardiomyopathy and hypertrophic cardiomyopathy: a population-based study in Olmsted Country, Minnesota, 1975-1984. Circulation 1989;80:564-570. [ Links ]
4. Ntusi NBA, Mayosi BM. The epidemiology of heart failure in Sub-Saharan Africa. Expert Rev Cardiovasc Ther 2009;7:169-180. [ Links ]
5. Schmidt MA, Michels VV, Edwards WD, Miller FA. Familial dilated cardiomyopathy. Am J Med Genet 1988;31:135-143. [ Links ]
6. Michels VV, Moll PP, Miller FA, et al. The frequency of familial dilated cardiomyopathy in a series of patients with idiopathic dilated cardiomyopathy. N Engl J Med 1992;326:77-82. [ Links ]
7. Keeling PJ, Gang Y, Smith G, et al. Familial dilated cardiomyopathy in the United Kingdom. Heart 1995;73:417-421. [ Links ]
8. Burkett EL, Hershberger RE. Clinical and genetic issues in familial dilated cardiomyopathy. J Am Coll Cardiol 2005;45:969-981. [ Links ]
9. Morita H, Seidman J, Seidman CE. Genetic causes of human heart failure. J Clin Invest 2005;115:518-526. [ Links ]
10. Finsterer J, Stollberger C. Cardiac involvement in primary myopathies. Cardiology 2000;94:1-11. [ Links ]
11. Michels VV, Driscoll DJ, Miller FA, et al. Progression of familial and non-familial dilated cardiomyopathy: long term follow up. Heart 2003;89:757-761. [ Links ]
12. Mayosi BM, Somers K. Cardiomyopathy in Africa: heredity versus environment. Cardiovasc J S Afr 2007;18:175-179. [ Links ]
13. Mestroni L, Maisch B, McKenna WJ, et al. Guidelines for the study of familial dilated cardiomyopathies. Eur Heart J 1999;20:93-102. [ Links ]
14. Evans W. Familial cardiomegaly. Br Heart J 1949;11:68-82. [ Links ]
15. Judge DP. Use of genetics in the clinical evaluation of cardiomyopathy. J Am Med Assoc 2009;302:2471-2476. [ Links ]
16. Van Spaendonck-Zwarts KY, van Tintelen JP, van Veldhuisen DJ, et al. Peripartum cardiomyopathy as a part of familial dilated cardiomyopathy. Circulation 2010;121:2169-2175. [ Links ]
17. Fragola PV, Autore C, Picelli A, et al. Familial idiopathic dilated cardiomyopathy. Am Heart J 1988;115:912-914. [ Links ]
18. Griffin M, Hernandez A, Martin T, et al. Dilated cardiomyopathy in infants and children. J Am Coll Cardiol 1988;11:139-144. [ Links ]
19. McKenna C, Codd M, McCann H, et al. Idiopathic dilated cardiomyopathy: familial prevalence and HLA distribution. Heart 1997;77:549-552. [ Links ]
20. Grünig E, Tasman JA, Kücherer H, et al. Frequency and phenotypes of familial dilated cardiomyopathy. J Am Coll Cardiol 1998;31:186-194. [ Links ]
21. Baig MK, Goldman JH, Caforio AP, et al. Familial dilated cardiomyopathy: cardiac abnormalities are common in asymptomatic relatives and may represent early disease. J Am Coll Cardiol 1998;31:195-201. [ Links ]
22. Coughlin SS, Comstock GW, Baughman KL. Descriptive epidemiology of idiopathic dilated cardiomyopathy in Washington County, Maryland 1975-1991. J Clin Epidemiol 1993;46:1003-1009. [ Links ]
23. Towbin JA, Lowe AM, Colan SD, et al. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA 2006;296:1867-1876. [ Links ]
24. Sliwa K, Damasceno A, Mayosi BM. Epidemiology and etiology of cardiomyopathy in Africa. Circulation 2005;112:3577-3583. [ Links ]
25. Moretti M, Merlo M, Barbati G, et al. Prognostic impact of familial screening in dilated cardiomyopathy. Eur J Heart Fail 2010;12(9):922-927. [ Links ]
26. Michels VV, Driscoll DJ, Miller FA Jr. Familial aggregation of idiopathic dilated cardiomyopathy. J Am Coll Cardiol 1985;55:1232-1233. [ Links ]
27. Mestroni L, Rocco C, Gregori D, et al. Familial dilated cardiomyopathy: evidence for phenotypic and genetic heterogeneity. J Am Coll Cardiol 1999;34:181-190. [ Links ]
28. Marenberg ME, Risch N, Berkman LF, et al. Genetic susceptibility to death from coronary heart disease in a study of twins. N Engl J Med 1994;330:1041-1046. [ Links ]
29. Ahmad F, Seidman JG, Seidman CE. The genetic basis for cardiac remodeling. Annu Rev Genomics Hum Genet 2005;6:185-216. [ Links ]
30. Khogali SS, Mayosi BM, Beattie JM, et al. A common mitochondrial DNA variant associated with susceptibility to dilated cardiomyopathy in two different populations. Lancet 2001;357:1265-1267. [ Links ]
31. Du Preez J, Matolweni LO, Greenberg J, et al. The alpha(2C) Del322-325 adrenergic receptor polymorphism is not associated with heart failure due to idiopathic dilated cardiomyopathy in black Africans. Cardiovasc J Afr 2008;19:15-16. [ Links ]
32. Seliem MA, Mansara KB, Palileo M, et al. Evidence for autosomal recessive inheritance of infantile dilated cardiomyopathy: studies from the eastern province of Saudi Arabia. Pediatr Res 2000;48:770-775. [ Links ]
33. Murphy RT, Mogensen J, Shaw A, et al. Novel mutation in cardiac troponin I in recessive idiopathic dilated cardiomyopathy. Lancet 2004;363:371-372. [ Links ]
34. Muntoni F, Cau M, Ganau A, et al. Brief report: Deletion of the dystrophin muscle-promoter region associated with X-liked dilated cardiomyopathy. N Engl J Med 1993;329:921-925. [ Links ]
35. Franz WM, Muller M, Muller OJ, et al. Association of nonsense mutation of dystrophin gene with disruption of sarcoglycan complex in X-linked dilated cardiomyopathy. Lancet 2000;355:1781-1785. [ Links ]
36. Hoogerwaard EM, Bakker E, Ippel PF, et al. Signs and symptoms of Duchenne muscular dystrophy and Becker muscular dystrophy among carriers in the Netherlands: a cohort study. Lancet 1999;353:2116-2119. [ Links ]
37. Palmucci L, Mongini T, Chiado-Piat L, et al. Dystrophinopathy expressing as either cardiomyopathy or Becker dystrophy in the same family. Neurology 2000;54:529-530. [ Links ]
38. Arbustini E, Morbini P, Pilotto A, et al. Familial dilated cardiomyopathy: from clinical presentation to molecular genetics. Eur Heart J 2000;21:1825-1832. [ Links ]
39. Hershberger RE, Hanson E, Jakobs PE, et al. A novel lamin A/C mutation in a family with dilated cardiomyopathy, prominent conduction system disease, and need for permanent pacemaker implantation. Am Heart J 2002;144:1081-1086. [ Links ]
40. Watkins DA, Hendricks N, Shaboodien G, et al. Clinical features, survival experience, and profile of plakophylin-2 gene mutations in participants of the Arrhythmogenic Right Ventricular Cardiomyopathy Registry of South Africa. Heart Rhythm 2009;6:S10-S17. [ Links ]
Accepted 21 March 2011.
Corresponding author: B M Mayosi (bongani.mayosi@uct.ac.za)














