Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.101 n.4 Pretoria Apr. 2011
SAMJ FORUM
HEALTH POLICY
Parenteral artesunate access programme aims at reducing malaria fatality rates in South Africa
E Visser Kift; T Kredo; K I Barnes
Parenteral artesunate should be used in preference to quinine for the treatment of severe malaria, given its significant mortality and safety benefits. As the product has not yet been registered for use in South Africa, the Parenteral Artesunate Access Programme has been launched to reduce malaria-related mortality. Severe malaria is a medical emergency that requires prompt treatment to prevent death, which occurs in 10 -50% of patients.1 Based on high-quality evidence, the World Health Organization (WHO) now strongly recommends intravenous (IV) artesunate in preference to IV quinine for the treatment of severe malaria in adults.2
Mortality and safety benefit
Artesunate, an artemisinin derivative, is highly effective in the treatment of malaria owing to its rapid parasite clearance, broad stage specificity and easy, safe administration compared with quinine.
The South-East Asian Quinine Artesunate Malaria Multicentre Randomised Controlled Trial (SEAQUAMAT) compared parenteral artesunate and quinine in 1 461 patients with severe Plasmodium falciparum malaria with death as the primary endpoint. The mortality rate was 15% in the artesunate arm compared with 22% in the quinine arm, with an absolute mortality risk reduction between study sites ranging from 5 -9%. Therefore, the numbers needed to treat to save one life ranged from 11 -20 patients. Treatment with artesunate was well tolerated, whereas quinine was associated with hypoglycaemia (relative risk (RR) 3.2, 1.3 -7.8; p=0.009).3 A Cochrane systematic review that informed the WHO treatment guidelines favoured the use of IV artesunate over quinine, with a 38% decrease in the risk of death (RR 0.62, 95% confidence interval (CI) 0.51 -0.75; 1 938 participants, 6 trials).4
The challenge
The Global Health Malaria Elimination Group has set the ambitious goal of eradicating malaria from the planet by 2050.5 Together with Namibia, Botswana and Swaziland, South Africa supports the WHO Roll Back Malaria (RBM) initiative in Africa and aims to eliminate malaria by 2018. South Africa has made exceptional progress in malaria control over the past decade, decreasing the number of notified malaria cases by 90%, from 61 934 in 2000 to 6 040 in 2009 (Department of Health Directorate of Malaria and other Vector Borne Diseases, unpublished data). Currently, South Africa has 0.71 malaria cases per 1 000 population at risk, and has therefore successfully moved from the effective control to the pre-elimination phase on the malaria elimination continuum. Yet the national malaria case fatality rate has remained essentially unchanged over the last decade and is currently 0.76%, well above the WHO target of 0.5%.6
The strategy
The Parenteral Artesunate Access Programme was launched in South Africa in January 2010. The parenteral artesunate used in all the above clinical trials is manufactured by Guilin Pharmaceuticals in China but is not yet registered for use in South Africa. The Malaria Advisory Group had motivated for a parenteral artesunate access programme since 2007. In June 2009, the Medicines Control Council (MCC) approved the use of parenteral artesunate in patients 12 years and older with severe malaria, on a named-patient basis under Section 21 of the Medicines and Related Substances Act. Regulatory authorities in the UK, USA, the EU, Australia and Canada have similar access programmes for the use of parenteral artesunate. The Parenteral Artesunate Access Programme secretariat is based at the University of Cape Town's Division of Clinical Pharmacology, and central pharmacy at Groote Schuur Hospital.
Ensuring drug quality
Artesunate was procured from Guilin Pharmaceuticals, who received WHO pre-qualification as operating in compliance with WHO Good Manufacturing Practice (GMP) in November 2010. Importation was authorised by the MCC and quality assurance performed by the MCC-approved Research Institute for Industrial Pharmacy, Potchefstroom University and the Mahidol Oxford Research Unit in Thailand.
Preliminary programme results
To date, 22 sentinel hospital sites have been enrolled in the access programme, trained and provided with parenteral artesunate stock. For each eligible patient, informed consent and MCC approval must be obtained, which is facilitated by the Parenteral Artesunate Access Programme secretariat. Case record forms are provided to encourage optimal assessment and monitoring of patients with severe malaria. Strict drug accountability is needed for the MCC to authorise ongoing access to parenteral artesunate.
To date, 92 patients (65 male) with a median age of 36 years (range 14 -79 years) at 16 hospitals in 6 provinces have received IV artesunate. Almost half (46%) of these patients were treated in the malaria-risk areas of Limpopo, Mpumalanga and KwaZulu-Natal, 33% in the Western Cape and 20% in Gauteng. This distribution of drug use is surprising as the largest number of malaria cases is reported in Gauteng,6 followed by Limpopo and Mpumalanga (Department of Health Directorate of Malaria and other Vector Borne Diseases, unpublished data).
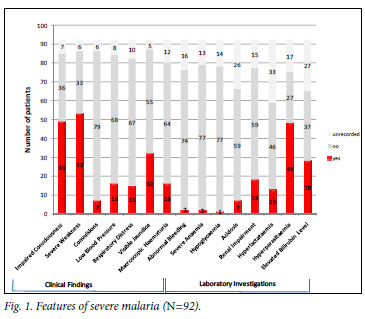
Of the 78 patients for whom we received reports on treatment outcome, 63 were well on discharge, 9 were not fully recovered (renal dysfunction or co-morbidities persisting) and 5 (6.4%) had died. There was a significant difference in the median (IQR) number of presenting complications between those who died and survived (5 (4 -6) v. 3 (2 -4); p=0.03). Fig. 1 shows the features of severe malaria present at diagnosis. Appropriate laboratory investigations were not recorded for all patients. Of the recorded complications, acidosis (p<0.0001), hyperlactataemia (p=0.007) and visible jaundice (p=0.04) were associated with death. No adverse events or serious adverse events have been reported to date.
The way forward
The preliminary data describe the effective and safe use of IV artesunate in South African adults, which supports continued Section 21 access until registered for use. Results recently released in the Lancet show that similar mortality reductions can be achieved in African children, with a relative mortality reduction of 22.5% (95% CI 8.1 -36.9; p=0.0231) and a corresponding number needed to treat of 41 children (95% CI 25 -112) to prevent one death.7 Based on these findings, an application for extending the parenteral artesunate access programme to children was recently approved by the MCC.
Success in reducing malaria-related morbidity and mortality in South Africa requires that increasing numbers of adult and paediatric severe malaria patients access the best available treatment. Parenteral artesunate is currently only available via the special access initiative. Hospitals interested in participating in this access programme can contact the corresponding author or secretariat (Marilyn Solomons, e-mail marilyn. solomons@uct.ac.za, phone 021 406 6355) for further details.
The authors gratefully acknowledge the guidance and support provided by the Medicines Control Council, Department of Health Directorate of Malaria and other Vector Borne Diseases, National Malaria Advisory Group, Provincial Malaria Control Programme Managers, participating hospital staff, Lesley Workman (Data Management) and the Parenteral Access Programme secretariat (Marilyn Solomons and Faikah Simons).
1. Day N, Dondorp AM. The management of patients with severe malaria. Am J Trop Med Hyg 2007;77(6):29-35. [ Links ]
2. World Health Organization Guidelines for the Treatment of Malaria, 2nd ed. Geneva: World Health Organization, 2006. http://whqlibdoc.who.int/publications/2010/9789241547925_eng.pdf (accessed 29 December 2010). [ Links ]
3. South East Asian Quinine Artesunate Malaria Trial (SEAQUAMAT) group. Artesunate versus quinine for treatment of severe falciparum malaria: a randomized trial. Lancet 2005;366:717-725. [ Links ]
4. Jones KL, Donegan S, Lalloo DG. Artesunate versus quinine for treating severe malaria. Cochrane Database of Systematic Reviews 2007, Issue 4. Art. No.: CD005967. DOI:10.1002/14651858.CD005967. pub2. (review update 2010, Issue 1. [ Links ])
5. Feachem RGA, Malaria Elimination Group. Shrinking the Malaria Map: A Guide on Malaria Elimination for Policy Makers. http://www.malariaeliminationgroup.org/sites/default/files/fileuploads/AGuideonMalariaEliminationforPolicyMakers.pdf (accessed 29 December 2010). [ Links ]
6. Weber IB, Baker L, Mnyaluza J, et al. The burden of imported malaria in Gauteng Province. S Afr Med J 2010;100:300-303. [ Links ]
7. Dondorp AM, Fanello CI, Hendriksen ICE, et al. Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomized trial. Lancet 2010;376:1647-1657. [ Links ]
Accepted 21 January 2011.
E Visser-Kift and K I Barnes are based at the Division of Clinical Pharmacology, Department of Medicine, University of Cape Town, where T Kredo was previously employed. T Kredo is currently affiliated to the South African Cochrane Centre, Medical Research Council, Cape Town.
Corresponding author: K Barnes (karen.barnes@uct.ac.za)














