Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
SAMJ: South African Medical Journal
versión On-line ISSN 2078-5135
versión impresa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.101 no.2 Pretoria feb. 2011
ORIGINAL ARTICLES
Immunogenicity and safety of an acellular pertussis, diphtheria, tetanus, inactivated poliovirus, Hib-conjugate combined vaccine (PentaximTM) and monovalent hepatitis B vaccine at 6, 10 and 14 weeks of age in infants in South Africa
Shabir Ahmed MadhiI; Clare CutlandI; Stephanie JonesI; Michelle GroomeI; Esteban OrtizII
IMD, Vaccine Preventable Diseases, Department of Science and Technology/National Research Foundation, and MRC Respiratory and Meningeal Pathogens Research Unit, University of the Witwatersrand, Johannesburg
IIMD, Sanofi Pasteur, Lyon, France
ABSTRACT
OBJECTIVE: To assess the immunogenicity and safety data for a pentavalent combination vaccine containing acellular pertussis, inactivated poliovirus, and Haemophilus influenzae (Hib) polysaccharide-conjugate antigens.
METHODS: A DTaP-IPV//PRP~T vaccine (PentaximTM) was given at 6, 10 and 14 weeks of age to 212 infants in South Africa. Monovalent hepatitis B vaccine was given concomitantly.
Immunogenicity was assessed using seroprotection and seroconversion rates; safety was assessed by monitoring for solicited injection site and systemic adverse events, and follow-up monitoring for unsolicited adverse events and serious adverse events.
RESULTS: Immunogenicity was high for each vaccine antigen, and similar to a reference study done in France using a similar (2, 3 and 4 months of age) administration schedule. After the third dose, 94.6% of participants had anti-PRP >0.15 µg/ml. The anti-PRP geometric mean antibody titre (GMT) was 2.0 µg/ml. The seroprotection rates for diphtheria and tetanus (>0.01 IU/ml), poliovirus types 1, 2 and 3 (>8 1/dil U) and hepatitis B were all 100%. Anti-polio GMTs were very high, 1 453, 1 699 and 2 398 (1/dil U) for types 1, 2 and 3, respectively. The seroconversion/vaccine response rates to pertussis antigens (4-fold increase in antibody concentration) were 97.5% for PT and 83.9% for FHA.
CONCLUSIONS: The DTaP-IPV//PRP~T vaccine was highly immunogenic at 6, 10 and 14 weeks of age in infants in South Africa, was compatible with the monovalent hepatitis B vaccine, and was well tolerated.
Vaccines are cost-effective public health tools to prevent and control infectious diseases. Their routine use has had enormous impact on diseases such as tetanus, diphtheria, pertussis, poliomyelitis and Haemophilus influenzae type b (Hib).1-5 Vaccines combining inactivated whole-cell Bordetella pertussis antigens with diphtheria and tetanus toxoids (DTwP) have been used for over 60 years and have been central to the World Health Organization (WHO) Expanded Program of Immunization (EPI) since 1974.
Safety and reactogenicity concerns of whole-cell pertussis (wP) vaccines led to the development of acellular pertussis (aP) vaccines, comprising purified B. pertussis antigens. These aP vaccines are better tolerated than wP vaccines, their protective efficacy has been shown in trials, and their use is supported by the WHO.2,6-10 aP vaccines are used in widespread national immunisation programmes and have been introduced in South Africa through a pentavalent combined vaccine.
The Sabin oral polio vaccine (OPV) has been successful in eliminating poliomyelitis, although replication of live attenuated polioviruses following vaccination can generate mutated viruses with renewed neurovirulence: vaccine-associated paralytic poliomyelitis (VAPP) and vaccine-derived polioviruses (VDPVs) are rare risks of OPV.11,12 In South Africa, polio vaccine-derived viruses have been detected in stool specimens of immunodeficient children,11 and in sewage and river water.13 Once wild poliovirus transmission has been interrupted OPV will be the only cause of poliomyelitis. In this context the use of inactivated poliovirus vaccines (IPVs) is important, there being no associated risk of VAPP or VDPVs.
The number of vaccines licensed and recommended for use in infants and children continues to increase. Incorporating multiple individual vaccines into combinations can simplify vaccine administration programmes and the inclusion of new antigens into immunisation schedules. Combination vaccines are an important advance in delivering vaccines to infants, helping to achieve high vaccination coverage and minimising costs.14-16
We assessed the immunogenicity and safety of the DTaP-IPV//PRP~T vaccine given with concomitant hepatitis B vaccination at 6, 10 and 14 weeks of age (EPI schedule) in South Africa.
Methods
Study design and participants
This was a phase III, open clinical trial at Chris Hani Baragwanath Hospital, Johannesburg. Participants received three doses of DTaP-IPV//PRP~T combined vaccine and of monovalent recombinant hepatitis B vaccine following the EPI schedule of administration (6, 10 and 14 weeks of age) (clinicaltrials.gov ID NCT00254969). The protocol was approved by the University of the Witwatersrand Human Subjects Research Ethics Committee and the Medicines Control Council (MCC). Parents or legal guardians gave written informed consent before trial inclusion.
Healthy full-term (>37 weeks) infants with a birth weight >2 500 g were eligible to participate and were sequentially screened and enrolled during the first 24 hours of life. Infants were excluded at screening or before the first vaccination at 6 weeks of age if they had already received a dose of OPV or if the mother was known to be seropositive for hepatitis B virus or HIV. In South Africa a dose of OPV is routinely given at birth, but for participating infants the first polio vaccination (IPV) was given at 6 weeks of age. At the time no published clinical data were available for this combined vaccine given at 6, 10 and 14 weeks of age. Data from a study using the same vaccine and a similar (2, 3 and 4 months of age) schedule in France were therefore used as a historical reference.17
Major exclusion criteria were acute febrile illness on the day of screening; any vaccination except BCG before trial participation; participation/planned participation in another clinical trial; receipt of blood or blood products; thrombocytopenia/bleeding disorder contraindicating intramuscular vaccination; a history of seizures; congenital/acquiredimmunodeficiencyorimmunosuppressivetherapy; known systemic hypersensitivity to any of the vaccine components; or a history of a life-threatening reaction to the trial vaccine or a vaccine containing the same substances. Infants who were screened and not included were referred to their local clinic for care and were offered vaccinations according to the national recommendations.
Vaccines
Each 0.5 ml dose of the combined DTaP-IPV//PRP~T vaccine (PentaximTM, Sanofi Pasteur, France, batch number Z2044-1 contained >30 IU diphtheria toxoid, >40 IU tetanus toxoid, 25 µg pertussis toxoid, 25 µg filamentous haemagglutinin, 40 U inactivated type 1 poliovirus, D antigen, 8 U inactivated type 2 poliovirus, D antigen, 32 U inactivated type 3 poliovirus, D antigen, and 10 µg H. influenzae type b polysaccharide covalently bound to tetanus toxoid (PRP~T). The PRP~T antigen was supplied as a freeze-dried powder and was rehydrated with an injectable suspension of DTaP-IPV vaccine at the time of injection. The combined vaccine was administered intramuscularly aseptically into the anterolateral aspect of the right upper thigh. The recombinant hepatitis B vaccine (Heberbiovac HB®, Biovac South Africa Ltd) was commercially available in South Africa at the time of the study. Each 0.5 ml dose contained 10 µg of recombinant hepatitis B surface antigen (HBsAg). The hepatitis B vaccine was administered intramuscularly aseptically into the anterolateral aspect of the left upper thigh.
Safety and reactogenicity
Participants were monitored for adverse reactions in the 30 minutes following each vaccine injection. Parents or legal guardians recorded time of onset, duration and severity of solicited injection site (tenderness, redness, swelling) and systemic (axillary temperature >37.4º C, vomiting, abnormal crying, drowsiness, loss of appetite, irritability) reactions on diary cards for the following 7 days. All solicited events were considered to be related to the vaccination, and any event related to vaccination was termed a 'reaction'. Unsolicited adverse events were recorded from each vaccination until the following study visit. Serious adverse events (SAEs) were recorded throughout the trial and for 6 months after the final vaccination.
Serology
Blood samples were taken before the first dose and 1 month after the third dose. Immunological assays were performed at the Sanofi Pasteur Global Clinical Immunology Platform Laboratory in the USA, except for PRP, which was analysed by the Health Protection Agency, UK, using an enzyme-linked immunosorbent assay (ELISA). Antidiphtheria and anti-tetanus, anti-PT and anti-FHA antibody titres were also measured by ELISA; anti-HBsAg titres were measured by VITROS chemoluminescence (ECi)/ECiQ Immunodiagnostic System; and anti-poliovirus type 1, 2 and 3 antibody titres (1/dil) were measured by microneutralisation. The pre-defined antibody levels for seroprotection (SP) were: anti-diphtheria >0.01 IU/ml; anti-tetanus >0.01 IU/ml; anti-poliovirus >8 reciprocal dilution (1/dil); anti-PRP >0.15 µg/ml; and anti-HBsAg >10 mIU/ml. Since there are no accepted correlates of seroprotection for pertussis antibodies, seroconversion (SC) for anti-PT and anti-FHA was defined as a >4-fold increase in antibody concentration after primary vaccination.
Statistical methods
Our main objective was to determine the seroprotection rates for diphtheria, tetanus, poliovirus types 1, 2 and 3, and PRP and the seroconversion rates for PT and FHA 1 month after the three-dose primary vaccination. We also determined the hepatitis B seroprotection rate, antibody titres to all vaccine antigens following priming, and the safety and reactogenicity of the study vaccines. The statistical analysis was descriptive. The sample size was chosen to give sufficient precision to allow a non-inferential comparison, based on 95% confidence intervals (CIs), with the results of a previous study in France in 212 infants with the same combined vaccine given in a similar administration schedule.17 The sample size was therefore set at 212 participants, ensuring at least 180 evaluable participants assuming a 15% drop-out rate.
SP and SC rates were calculated with their 95% CIs by the Clopper-Pearson exact binomial method. Geometric mean antibody titres (GMTs) were calculated with their 95% CIs using the normal approximation method. Reverse cumulative distribution curves (RCDCs) were constructed for each antibody response. Infants who received at least one dose of vaccine and had at least one antibody titration available after primary immunisation and no protocol deviations were included in the immunogenicity analysis. All participants who received at least one dose of study vaccine were included. To evaluate reactogenicity, the number and percentage of participants with solicited symptoms after each dose of vaccine were calculated.
Results
Participant disposition
Of 300 participants, 88 (29.3%) discontinued between consent being given at birth and the administration of the first dose of vaccine at 6 weeks of age (Fig. 1). The main reasons for non-inclusion were voluntary withdrawal not due to an adverse event (47, 53.4%) and loss to follow-up (26, 29.5%). Six (6.8%) of the 88 discontinued participants were not compliant with the study protocol, and 9 (10.2%) were screened but not included because the planned number of participants had been reached and received the standard of care and vaccinations according to the national recommendations and their physician's clinical judgement.
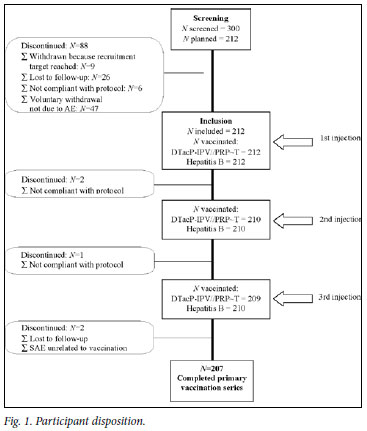
As planned, 212 participants received the first dose of vaccine; 50.5% were male, the mean age was 43.2 days (standard deviation (SD) 1.6 days) (approximately 6.2 weeks), and the mean weight was 3 200 g (SD 400 g). The primary vaccination phase was completed by 207 participants (97.6%) (Fig. 1). Three of the 5 who discontinued were withdrawn because of non-compliance with the protocol, one was lost to follow-up, and one discontinued because of an SAE (fatal dehydration and gastro-enteritis unrelated to vaccination).
Immunogenicity
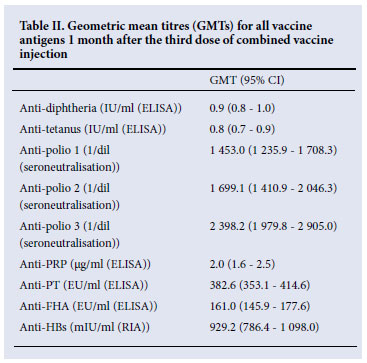
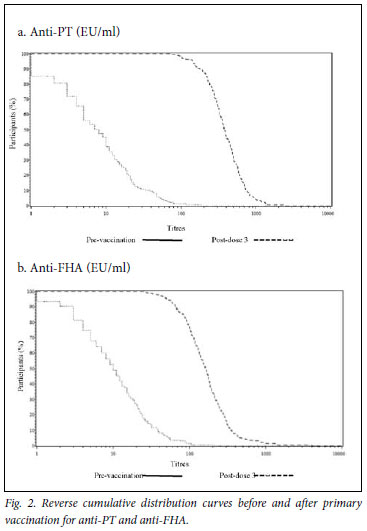
The SP/SC rates were high for each antigen after completion of the three-dose primary series and were similar to the historical data (Table I). All participants were seroprotected against diphtheria (>0.01 IU/ml), tetanus (>0.01 IU/ml), poliovirus types 1, 2, and 3 (>8 (1/dil)) and hepatitis B (>10 mIU/ml); 94.6% of participants had anti-PRP titres >0.15 µg/ml. The SC rates (>4-fold increase in antibody titres) for pertussis antigens were 97.5% and 83.9% for PT and FHA, respectively. Overall, the SP/SC rates were similar to the reference study. Post-priming GMTs were high (Table II). There were marked increases in anti-PT and anti-FHT GMTs from pre-dose 1 to post-dose 3. The GMT ratios (GMTRs) were 53.5 for PT and 16.8 for FHA. All study participants had post-priming anti-PT and anti-FHA >25 EU/ml. Anti-PT and FHA antibody titres are graphically described using RCDCs in Fig. 2, which shows strong, linear increases in antibody titre following primary series vaccination.
Reactogenicity and safety Solicited reactions
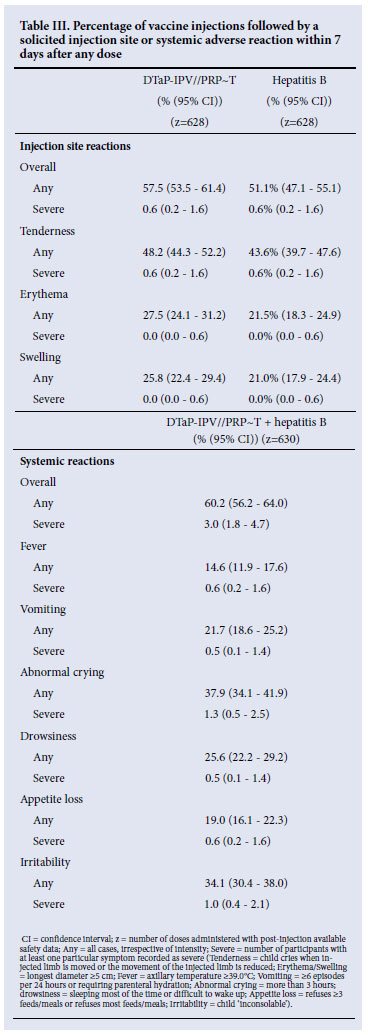
Table III summarises solicited symptoms after any dose of study vaccine. Reactogenicity was low for the combined and the hepatitis B vaccine. Most solicited injection site reactions occurred within 3 days after vaccination, were mild to moderate in severity, and resolved within 8 days. The most frequent injection site reactions were tenderness, swelling and erythema, with severe tenderness after only 0.6% of doses (4/628); severe swelling or erythema were not reported. The most frequently reported solicited systemic reactions were abnormal crying and irritability. Severe systemic reactions were reported after 0.5 -1.3% of injections.
Unsolicited reactions
Two participants (0.9%) reported unsolicited injection site reactions. One experienced tenderness and erythema at the injection site of the combined vaccine and tenderness at the hepatitis B vaccine injection site that started more than 8 days after the second injection. The other had bruising at the combined vaccine injection site. Unsolicited systemic events were reported by 155 participants (73.1%). Most were diagnoses commonly seen in infants. Only 8 of the 155 participants reported an unsolicited systemic event assessed as related to vaccination (diarrhoea, gastro-enteritis, lower respiratory tract infection, otitis media, rhinitis, tonsillitis and generalised rash), but none was severe. No cases of hypotonic hyporesponsive episode or seizure were reported.
Twenty participants experienced 22 SAEs; none was considered to be related to vaccination. Of the 2 deaths, one resulted from gastro-enteritis diagnosed about a month after the third dose, followed, during hospitalisation, by dehydration with acidosis and herbal intoxication. The second fatality resulted from gastro-enteritis diagnosed about 4 months after the third dose. The remaining 18 participants with SAEs (bronchopneumonia, gastro-enteritis, bronchiolitis, epilepsy) recovered with treatment.
There was no case of litigation for any solicited reaction, unsolicited event or SAE.
Discussion
This study assessed the immunogenicity and safety of a DTaP-IPV//PRP~T combination vaccine for primary vaccination at 6, 10 and 14 weeks of age, given concomitantly with a stand-alone recombinant hepatitis B vaccine to infants in South Africa. This combined vaccine was first licensed in 1997 in Europe and contains antigens that are well known as stand-alone vaccines. The PRP~T component is licensed as ActHIBTM and the IPV component as Imovax PolioTM.18
This was compared with historical data following a 2, 3 and 4 months administration schedule,17 which was used rather than 6, 10 and 14 weeks data from the Philippines19 and India20 because the latter were not available when our protocol was developed. The study therefore shows that the DTaP-IPV//PRP~T combined vaccine elicits strong immune responses following primary vaccination with the EPI-recommended 6, 10 and 14 weeks of age schedule, comparable with the historical data.
The safety and immunogenicity of the antigens included in the DTaP-IPV//PRP~T study vaccine have been shown in clinical studies in Europe, North and South America, Africa and Asia.19-24 The pertussis antigen immunogenicity data from 36 trials of this aP formulation in combination vaccines conducted in 17 countries have also been reviewed.25 In those trials, the GMTs ranged from 49 to 184 EU/ml for anti-PT and from 95 to 293 EU/ml for anti-FHA following primary immunisation. The addition of other valences, i.e. IPV and PRP~T, or concomitant administration of hepatitis B did not affect the immunogenicity of the PT and FHA antigens included in the combination.
The long-term impact of the DTaP-IPV//PRP~T combined vaccine on pertussis incidence has been surveyed in Sweden over 10 years.26,27 Although the South African schedule differs from that in Sweden, we believe that these data are applicable to EPI schedules since high immunogenicity has been demonstrated in different primary vaccination regimens with this combined vaccine.25 Routine primary vaccination with aP vaccines, including PentaximTM, resulted in a marked decrease in pertussis incidence, with protection remaining high for 5 -7 years after the third dose.28
As South Africa was close to certification as polio free at the time of the study (its last case of polio caused by a wild-type polio virus occurred in 198929), omission of the OPV birth dose posed no risk to participants and did not pose ethical problems. Combined vaccines that include IPV are available in the South African National Immunisation Programme and the seroprotection achieved against all three poliovirus types in our study provides additional evidence for the immunogenicity of the IPV antigens included in the combination vaccine at 6, 10 and 14 weeks of age. The anti-poliovirus GMTs observed after primary vaccination were very high compared with clinical trials with this vaccine in other countries following several primary vaccination schedules.19-24 The high immunogenicity of the IPV in this study is of importance because of the vaccination schedule and for maintaining population immunity against poliomyelitis following elimination of wild poliovirus circulation and cessation of OPV.30 The inclusion of IPV in a DTaP combined vaccine ensures vaccination coverage as high as that for three doses of pertussis vaccine and avoids the additional vaccinations required if IPV were to be added to the schedule as a separate vaccine. Routine Hib vaccination has dramatically decreased the incidence of disease in many countries,1,3 including South Africa following its introduction in 1999.31 The combination of DTP antigens with PRP~T does not significantly alter the immunogenicity of efficacy of the Hib vaccine.32,33 In our study the anti-PRP antibody response >0.15 µg/ml in 94.6% of participants was comparable to the 98 -98.7% rates in the Philippine and Indian studies at 6, 10 and 14 weeks.19,20 The anti-PRP result is also similar to studies that used the same combined vaccine at 2, 3 and 4 months of age or 2, 4 and 6 months of age.19-24 There was no negative impact when the combined vaccine and the recombinant hepatitis B vaccine were given concomitantly at separate injection sites, as the immune responses were satisfactory and safety data were acceptable for use in this South African population. The hepatitis B vaccination programme in South Africa has markedly reduced HBsAg carriage in children younger than 5 years of age.34 Finally, compatibility with the EPI 6, 10 and 14 weeks of age schedule ensures the early protection of infants and avoids late administration of vaccines that might impair the success of child immunisation programmes.35,36
The DTaP-IPV//PRP~T combination and monovalent hepatitis B vaccines were well tolerated and had similar reactogenicity profiles, and their safety results reflect the good reactogenicity previously documented for aP-based combination vaccines.2,7
Conclusion
The DTaP-IPV//PRP~T combined vaccine (PentaximTM, an AcXim family vaccine) was highly immunogenic for all antigens when administered to infants following the 6, 10 and 14 weeks EPI schedule. The seroprotection and seroconversion rates were similar to historical data. The inclusion of IPV and PRP~T valences facilitates their addition to the national immunisation schedule with high coverage equivalent to that of three doses of DTP vaccine. The combined vaccine was compatible and well tolerated when given concomitantly with hepatitis B vaccine.
The authors thank the participating clinicians, the infants who participated and their parents. We acknowledge Clement Weinberger (Le Stylo Communications) and Andrew Lane for assistance with the manuscript preparation, Roy Fernando for data management and Valérie Bosch-Castells for the statistical analysis. Roy Fernando, Valérie Bosch-Castells and Andrew Lane are employees of Sanofi Pasteur.
The study was conducted with the financial support of Sanofi Pasteur, Lyon, France, and presented in part at the 13th International Congress on Infectious Diseases, June 2008, Kuala Lumpur, Malaysia.
References
1. Chandran A, Watt JP, Santosham M. Haemophilus influenzae vaccines. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. 5th ed. Philadelphia: Saunders Elsevier, 2008: 157-176. [ Links ]
2. Edwards K, Decker M. Pertussis vaccines. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. 5th ed. Philadelphia: Saunders Elsevier, 2008: 467-517. [ Links ]
3. Peltola H. Worldwide Haemophilus influenzae type b disease at the beginning of the 21st century: global analysis of the disease burden 25 years after the use of the polysaccharide vaccine and a decade after the advent of conjugates. Clin Microbiol Rev 2000;13(2):302-317. [ Links ]
4. Vitek CR, Wharton M. Diphtheria toxoid. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. 5th ed. Philadelphia: Saunders Elsevier, 2008: 139-155. [ Links ]
5. Wassilak SG, Roper MH, Kretsinger K, Orenstein WA. Tetanus toxoid. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. 5th ed. Philadelphia: Saunders Elsevier, 2008: 805-839. [ Links ]
6. Cherry JD. Comparative efficacy of acellular pertussis vaccines: an analysis of recent trials. Pediatr Infect Dis J 1997;16(4 Suppl):S90-S96. [ Links ]
7. Decker MD, Edwards KM, Steinhoff MC, Rennels MB, Pichichero ME, Englund JA. Comparison of 13 acellular pertussis vaccines: adverse reactions. Pediatrics 1995;96(3 Pt 2):557-566. [ Links ]
8. Edwards KM, Meade BD, Decker MD, et al. Comparison of 13 acellular pertussis vaccines: overview and serologic response. Pediatrics 1995;96(3 Pt 2):548-557. [ Links ]
9. Simondon F, Preziosi MP, Yam A, et al. A randomized double-blind trial comparing a two-component acellular to a whole-cell pertussis vaccine in Senegal. Vaccine 1997;15(15):1606-1612. [ Links ]
10. World Health Organization. Pertussis position paper. Wkly Epidemiol Rec 2005;80(4):31-39. [ Links ]
11. Vaccine-derived polioviruses - update. Wkly Epidemiol Rec 2006;81(42):398-404. [ Links ]
12. Kew OM, Sutter RW, de Gourville EM, Dowdle WR, Pallansch MA. Vaccine-derived polioviruses and the endgame strategy for global polio eradication. Annu Rev Microbiol 2005;59:587-635. [ Links ]
13. Pavlov DN. Poliovirus vaccine strains in sewage and river water in South Africa. Can J Microbiol 2006;52(8):717-23. [ Links ]
14. Edwards KM, Decker MD. Combination vaccines: hopes and challenges. Pediatr Infect Dis J 1994;13(5):345-347. [ Links ]
15. Obaro SK, Palmer A. Vaccines for children: policies, politics and poverty. Vaccine 2003;21(13-14):1423-1431. [ Links ]
16. Pichichero ME. New combination vaccines. Pediatr Clin North Am 2000;47(2):407-426. [ Links ]
17. Mallet E, Hoffenbach A, Salomon H, Blondeau C, Fritzell B. Primary immunization with combined, acellular DTaP-IPV-Act-HIB vaccine given at 2-3-4 or 2-4-6 months of age. Presented at the 14th Annual Meeting of the European Societies for Paediatric Infectious Diseases, Elisnore, Denmark, June 1996. Abstract 19. [ Links ]
18. United Nations pre-qualified vaccines: WHO list of vaccines for purchase by UN agencies. http://www.who.int/immunization_standards/vaccine_quality/PQ_vaccine_list_en/en/index.html (accessed 19 October 2010). [ Links ]
19. Capeding MR, Cadorna-Carlos J, Book-Montellano M, Ortiz E. Immunogenicity and safety of a DTaP-IPV//PRP~T combination vaccine given with hepatitis B vaccine: a randomized open-label trial. Bull World Health Organ 2008;86(6):443-451. [ Links ]
20. Dutta AK, Verghese VP, Pemde HK, Mathew LG, Ortiz E. Immunogenicity and safety of a pentavalent diphtheria, tetanus, acellular pertussis, inactivated poliovirus, Haemophilus influenzae type B conjugate combination vaccine (Pentaxim) with hepatitis B vaccine. Indian Pediatr 2009;46(11):975-982. [ Links ]
21. Carlsson RM, Claesson BA, Selstam U, et al. Safety and immunogenicity of a combined diphtheriatetanus-acellular pertussis-inactivated polio vaccine-Haemophilus influenzae type b vaccine administered at 2-4-6-13 or 3-5-12 months of age. Pediatr Infect Dis J 1998;17(11):1026-1033. [ Links ]
22. Kanra G, Silier T, Yurdakok K, et al. Immunogenicity study of a combined diphtheria, tetanus, acellular pertussis, inactivated poliomyelitis vaccine used to reconstitute a freeze-dried Haemophilus influenzae type b vaccine (DTaP-IPV//PRP-T) administered simultaneously with a hepatitis B vaccine at two, three and four months of life. Vaccine 1999;18(9-10):947-954. [ Links ]
23. Lagos R, Kotloff K, Hoffenbach A, et al. Clinical acceptability and immunogenicity of a pentavalent parenteral combination vaccine containing diphtheria, tetanus, acellular pertussis, inactivated poliomyelitis and Haemophilus influenzae type b conjugate antigens in two-, four-and six-month-old Chilean infants. Pediatr Infect Dis J 1998;17(4):294-304. [ Links ]
24. Mallet E, Fabre P, Pines E, et al. Immunogenicity and safety of a new liquid hexavalent combined vaccine compared with separate administration of reference licensed vaccines in infants. Pediatr Infect Dis J 2000;19(12):1119-1127. [ Links ]
25. Vidor E, Plotkin SA. Immunogenicity of a two-component (PT & FHA) acellular pertussis vaccine in various combinations. Hum Vaccin 2008;4(5):328-340. [ Links ]
26. Olin P, Hallander HO. Marked decline in pertussis followed reintroduction of pertussis vaccination in Sweden. Euro Surveill 1999;4(12):128-129. [ Links ]
27. Swedish Institute for Infectious Disease Control. Pertussis surveillance in Sweden with enhanced follow-up of cohorts immunized with acellular pertussis vaccines. 2009. Appendix 2. http://www.smittskyddsinstitutet.se/upload/Publikationer/11-y-report-app202-GSK.pdf (accessed 15 December 2010). [ Links ]
28. Gustafsson L, Hessel L, Storsaeter J, Olin P. Long-term follow-up of Swedish children vaccinated with acellular pertussis vaccines at 3, 5, and 12 months of age indicates the need for a booster dose at 5 to 7 years of age. Pediatrics 2006;118(3):978-984. [ Links ]
29. Pavlov DN, Van Zyl WB, Van Heerden J, et al. Prevalence of vaccine-derived polioviruses in stools of immunodeficient children in South Africa. J Appl Microbiol 2006;101(6):1367-1379. [ Links ]
30. Global Polio Eradication Initiative - Strategic Plan 2009 -2013. http://www.polioeradication.org/content/publications/PolioStrategicPlan09-13_Framework.pdf (accessed 19 October 2010). [ Links ]
31. von Gottberg A, de Gouveia L, Madhi SA, et al. Impact of conjugate Haemophilus influenzae type b (Hib) vaccine introduction in South Africa. Bull World Health Organ 2006;84(10):811-818. [ Links ]
32. Gold R, Scheifele D, Barreto L, et al. Safety and immunogenicity of Haemophilus influenzae vaccine (tetanus toxoid conjugate) administered concurrently or combined with diphtheria and tetanus toxoids, pertussis vaccine and inactivated poliomyelitis vaccine to healthy infants at two, four and six months of age. Pediatr Infect Dis J 1994;13(5):348-355. [ Links ]
33. Lagos R, Horwitz I, Toro J, et al. Large scale, postlicensure, selective vaccination of Chilean infants with PRP-T conjugate vaccine: practicality and effectiveness in preventing invasive Haemophilus influenzae type b infections. Pediatr Infect Dis J 1996;15(3):216-222. [ Links ]
34. Tsebe KV, Burnett RJ, Hlungwani NP, Sibara MM, Venter PA, Mphahlele MJ. The first five years of universal hepatitis B vaccination in South Africa: evidence for elimination of HBsAg carriage in under 5-year-olds. Vaccine 2001;19(28-29):3919-3926. [ Links ]
35. Clark A, Sanderson C. Timing of children's vaccinations in 45 low-income and middle-income countries: an analysis of survey data. Lancet 2009;373(9674):1543-1549. [ Links ]
36. Guerra FA. Delays in immunization have potentially serious health consequences. Paediatr Drugs 2007;9(3):143-148. [ Links ]
Accepted 12 November 2010.
Corresponding author: E Ortiz (esteban.ortiz@sanofipasteur.com)














