Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.101 n.2 Pretoria Feb. 2011
ORIGINAL ARTICLES
A bird's eye view of PMTCT coverage at two regional hospitals and their referral clinics in a resource-limited setting
Dhayendre MoodleyI; Jyothi SrikewalII; Lindiwe MsweliIII; Niren R MaharajIV
IPhD, Women's Health and HIV Research Unit, Department of Obstetrics and Gynaecology, Nelson R Mandela School of Medicine, University of KwaZulu-Natal, Durban
IIMB BCh, Women's Health and HIV Research Unit, Department of Obstetrics and Gynaecology, Nelson R Mandela School of Medicine, University of KwaZulu-Natal, Durban
IIIBA, Dip SC, Women's Health and HIV Research Unit, Department of Obstetrics and Gynaecology, Nelson R Mandela School of Medicine, University of KwaZulu-Natal, Durban
IVFCOG (SA), Women's Health and HIV Research Unit, Department of Obstetrics and Gynaecology, Nelson R Mandela School of Medicine, University of KwaZulu-Natal, Durban
ABSTRACT
BACKGROUND: While countries strengthen their health information systems, local health managers require alternative strategies to monitor their prevention of mother-to-child transmission (PMTCT) programmes to improve coverage and service delivery.
OBJECTIVE: To demonstrate the use of a postpartum audit to establish PMTCT coverage and programme deficiencies at hospitals and multiple primary health care facilities.
METHODS: A cross-sectional hospital-based medical chart audit of pregnant women admitted in labour to their regional hospital. Their antenatal hand-held medical records were added to a hospital-issued maternity chart that was used to record further obstetric and perinatal management during their hospital stay. Women recuperating in the postnatal wards up to 48 hours after delivery at two hospitals in KwaZulu-Natal participated. Data included their antenatal attendance, access to HIV counselling and testing (HCT), and access to nevirapine (NVP) for PMTCT.
RESULTS: Fifty-three clinics were indirectly evaluated as a result of the postpartum audit. All clinics provided HCT and the average HIV testing rate was 91% (range 40 -100); 15% (N=8) of these clinics with HIV testing rates of <80% were identified. The median frequency of NVP dispensing at 53 clinics was 87% (interquartile range 67 -100); among these 30% (N=16) with NVP dispensing frequencies of <80% were identified.
CONCLUSION: An exit survey by trained nurses at a maternity hospital can provide health services management with a quick estimate of antenatal and PMTCT coverage of multiple primary health facilities in a specified catchment area. Challenges in the PMTCT programme at primary health clinic and hospital levels were highlighted.
Despite strategies to eradicate mother-to-child transmission of HIV, two-thirds of the affected population in middle-and low-income countries have no access to basic interventions in prevention of mother-to-child transmission (PMTCT) services.1 To achieve a successful commitment to the UN General Assembly Special Session on HIV/AIDS (UNGASS) agreement on universal access to HIV-related care, 143 of the 149 low-and middle-income countries have reported their PMTCT coverage data.1,2
Measuring PMTCT coverage depends on routine programme data collected by in-country health management information systems. PMTCT data are routinely collected from antenatal and delivery registers at primary health clinics and are submitted as monthly or quarterly aggregates to the local information centres for onward reporting to higher management structures.
Such monitoring systems have been flawed, with incomplete or inaccurate measures of PMTCT service coverage, and are also not used by local stakeholders to improve health service delivery.3 While low-and middle-income countries strive to improve their information systems and obtain more reliable data, local health service managers require alternative strategies to monitor PMTCT programmes to improve PMTCT coverage.
Resource-and time-constrained managers need assistance to improve the coverage and quality of a more comprehensive PMTCT programme in their respective geographical areas. We used a single cross-sectional hospital-based monitoring tool to demonstrate its use in measuring antenatal and PMTCT coverage and identifying programme deficiencies at multiple primary health care facilities.
Methods
Pregnant women sought antenatal care at their respective primary health clinics in the Umlazi catchment area. When admitted in labour at their regional hospital, their antenatal hand-held medical records were added to a hospital-issued maternity chart that was used to record further obstetric and perinatal management during their hospital stay. We conducted a cross-sectional survey utilising a structured data extraction form among women recuperating in the postnatal wards within 48 hours of delivery at an urban and a peri-urban hospital in KwaZulu-Natal. Written informed consent was obtained from women recuperating after delivery, who resided in the hospital's catchment area and were willing to participate. Data were obtained from the maternal health records and from interviews with participants by research nurses. Antenatal and peripartum records were examined in the presence of participants to extract information regarding their: (i) antenatal clinic attendance; (ii) access to HIV counselling and testing (HCT); and (iii) access to nevirapine (NVP) for PMTCT prophylaxis. Information obtained from the maternity records was verified through an interview with the respective participant in addition to obtaining reasons for limited access to any of the listed services.
The hospitals each serve the population of a specific catchment area. Pregnant women living in these areas seek antenatal care at the primary health clinic closest to their residence, expect to deliver at the referred hospital for the catchment area, and return for postnatal and infant care at the primary health clinic.
The structured English data form, developed after considering the national PMTCT programme indicators and the content of maternity case records, was piloted among 10 women in each of two of the hospitals and revised to ensure unambiguous responses. Four trained research nurses conducted the survey at each of the hospitals. The data were captured and analysed using Epi Info version 6.0.
Ethical approval was obtained from the University of KwaZulu-Natal institutional review board (IRB), and permission to conduct the study was obtained from the respective health district management services.
Results
During the months August and September 2007, 1 525 women delivered at the two hospital facilities, among whom 805 (53%) women recuperating in the postnatal wards consented to participate. The participants were 414 (40%) of 1 033 women and 391 (79%) of 492 women who delivered at the urban and peri-urban hospitals, respectively. Women who delivered at the two hospitals were antenatal attendees at 53 primary health care facilities in the catchment areas. The hospitals serve as referral maternity hospitals for 31 and 22 primary health clinics, respectively.
Population characteristics
The mean age of women interviewed was 25 years (range 16 -44 years); 80 - 90% were unemployed, unmarried and did not live with their partner (Table I), 39.5% were primiparous, and the average gestational age at antenatal booking was 24 weeks (range 6 -39 weeks). The population demographics and antenatal characteristics were comparable in the two geographical areas (Table I).
Common reasons for registering at antenatal clinics after 28 weeks of pregnancy (N=234) included attendance at school or work (12%), feeling that the pregnancy was going well (8%), being refused antenatal access at a clinic (7%), being uninformed about the need for early registration (26%), and being unsure whether they were pregnant (11%); 36% did not provide a reason for registering late in pregnancy.
Access to HIV counselling and testing
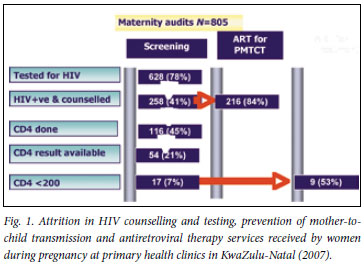
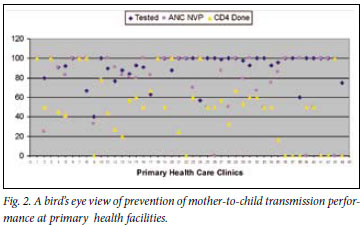
From the patient records, it was determined that 676 (84%) of the 805 women had a documented record of access to HCT; 48 (7%) who received pretest counselling refused HIV testing, and 258 (41%) of the 628 women who accepted testing tested positive (Fig. 1). The average HIV testing rate at all primary health care facilities was 91% (range 40 -100). Evidence of HCT documentation in maternity records was similar in the urban (85%) and peri-urban (86%) primary health clinics (odds ratio (OR) 1.06, 95% confidence interval (CI) 0.7 -1.59; p=0.86). Likewise, access to HCT services (80% v. 81%) and the HIV prevalence (36% v. 34%) among the women were comparable in the two geographical areas. Although not statistically significant, there was a trend towards a higher rate of refusal to test for HIV at the periurban clinics (8.3% v. 5.9%; OR 1.44, 95% CI 0.8 -2.7). All 53 clinics offered HCT and 8 (15%) clinics were identified with HIV testing rates of less than 80% (Fig. 2).
Access to PMTCT of HIV prophylaxis
There was no documentation of PMTCT prophylaxis in 33 (13%) of the maternity records and 10 (18.9%) clinics. This was significantly (p=0.002) more evident among women who attended clinics in the peri-urban area (OR 3.6, 95% CI 1.5 -8.8) (Table II). The median frequency of antenatal NVP dispensing at 43 of the primary health clinics attended by HIV-positive pregnant women was 87% (interquartile range (IQR) 67 - 100). Among these, 15 facilities (33%) were reported with antenatal NVP dispensing frequencies of less than 80%, 9 of which were also identified with sub-optimal (<75%) access to HCT services. One hundred and ninety-five HIV-positive pregnant women (76%) had NVP administered during labour at the delivery hospital. The urban clinics were more likely (p=0.002) to dispense NVP during antenatal attendance (OR 3.1, 95% CI 1.4 - 6.6).
Overall, considering all facilities 24% (N=64) of participants audited had not received any antiretroviral intervention according to national guidelines for PMTCT of HIV. One hundred and eighty-four HIV-exposed infants (68%) had documentation of receiving NVP before discharge.
Women's access to antiretroviral treatment
Of the 258 HIV-positive women, 116 (45%) had documentation that a CD4 count had been done and 54 (46%) received their results during the antenatal period (Fig. 1). Among those who received their results, 17 (31%) had a CD4 count of <200 cells/µl. Of women eligible for HAART, 8 (47%) initiated HAART during pregnancy; the remainder were given the standard single-dose NVP regimen. Two hundred and four of the HIV-positive women (79%) did not have a CD4 count performed or had not received their results during pregnancy, and 36 (84%) of the clinics were reported as having CD4 testing rates <80% (Fig. 2).
Discussion
We demonstrated the use of a structured questionnaire/data extraction form administered to postpartum women by nurses at two hospitals during a 1-month period to describe PMTCT coverage at 52 primary health clinics and highlighted deficiencies in maternity and HIV-related service provision.
Our findings demonstrate encouraging HIV counselling and testing (HCT) coverage. More than 80% of women surveyed had accessed HCT services during their antenatal visits at their primary health facilities. Of these, 90% had been tested for HIV, and 75% of the HIV-positive women received PMTCT prophylaxis. Notably, 24% of women had not received any PMTCT prophylaxis and one-third of exposed infants did not receive prophylaxis before hospital discharge.
The implementation and operational effectiveness of the PMTCT programme in South Africa have been studied.4-6 These have provided policy makers and other stakeholders in the public health sector with an understanding of challenges in the implementation of PMTCT programmes. However, guidance to local health service managers for ongoing monitoring of PMTCT coverage and quality of service delivery is limited.
Our monitoring system, using a combined chart audit/questionnaire at a single maternity hospital in a specified catchment area, offers health service managers a 'bird's eye view' of PMTCT coverage and an opportunity for district management to oversee maternal health services at multiple primary health clinics and quality of care at the hospital using minimum resources. This also enables targeting of facilities that demonstrate sub-optimal performance.
Other forms of monitoring are recognised as reliable sources of health service coverage. Antenatal HIV surveillance data have determined the proportion of pregnant women participating in PMTCT programmes.7 Geographical information systems (GIS) have been used to determine access to and utilisation of HIV services in Uganda.8 The GIS mapping is effective in identifying deficiencies in facility-based service delivery, and inequities in access to care in different geographical areas. A similar postnatal audit at two maternity hospitals in Thailand measured the coverage of services offered at the hospital alone and focused only on HIV-infected women.9
Our audit also highlighted deficiencies in service provision that should be investigated and targeted for improvement. The average gestational age at antenatal booking was 23 weeks, 66% booking between 14 and 28 weeks. This implies that dual ARV prophylaxis using AZT from 14 weeks is feasible in at least two-thirds of pregnant women and should ensure that women receive a minimum of 4 weeks of AZT antenatally.10 The frequency of antenatal visits ranged from 3 to 7 visits per participant, hence providing several opportunities for women to receive VCT and commence PMTCT prophylaxis.
Initiation of simple interventions in South Africa was challenging, its operational challenges manifested in poor uptake of HIV testing (56%), only 55% of women being given single-dose NVP, and only 50% of infants being followed up until 12 months and tested.11 These challenges in ensuring wide-scale implementation and quality assurance of PMTCT programmes in resource-limited countries are known to be due to human resource limitation at primary health clinics (2.3 health care workers per 1 000 population).12 Time, human resource and financial constraints can lead to inadequate support, supervision and monitoring of health services at primary health clinics.
The authors wish to thank the research nurses, facility management (PMMH and GJ Crookes) and the data management team for their invaluable contribution towards the successful implementation of this project.
References
1. World Health Organization. UNAIDS. UNICEF. Towards universal access: scaling up priority HIV/AIDS interventions in the health sector: progress report 2008. www.who.int/hiv/pub/towards_universal_access_report_2008.pdf (accessed April 2010). [ Links ]
2. UN General Assembly Special Session on HIV/AIDS (UNGASS). Declaration of Commitment on HIV/AIDS. New York: UN, 2001. www.unaids.org/en/Goals/UNGASS/default.asp (accessed April 2010). [ Links ]
3. Mate KS, Bennet B, Mphatswe W, Barker P, Rollins N. Challenges for routine health system data management in a large public programme to prevent mother-to-child HIV transmission in South Africa. PLoS ONE 2009;4(5):e5483 [ Links ]
4. Nkonki LL, Doherty TM, Hill Z, Chopra M, Schaay N, Kendall C. Missed opportunities for participation in prevention of mother to child transmission programmes: Simplicity of nevirapine does not necessarily lead to optimal uptake, a qualitative study. AIDS Research and Therapy 2007;4:27-33. [ Links ]
5. Coetzee D, Hilderbrand K, Boulle A, Draper B, Abdullah F, Goemaere E. Effectiveness of the first district-wide programme for the prevention of mother-to-child transmission of HIV in South Africa. Bull World Health Organ 2005;83(7):489-494. [ Links ]
6. Colvin M, Chopra M, Doherty T, et al. Operational effectiveness of single-dose nevirapine in preventing mother-to-child transmission of HIV. Bull World Health Organ 2007;85(6):466-473. [ Links ]
7. Bolu O, Anand A, Swartzendruber A, et al. Utility of antenatal HIV surveillance data to evaluate prevention of mother-to-child HIV transmission programs in resource-limited settings. Am J Obstet Gynecol 2007;197(3 Suppl):S17-25. [ Links ]
8. Chamia DD, Olu O, Wanyana J, et al. Geographical information system and access to HIV testing, treatment and prevention of mother-to-child transmission in conflict affected Northern Uganda. Conflict and Health 2007;1:12. [ Links ]
9. Teeraratkul A, Simonds RJ, Asavapiriyanont S, et al. Evaluating programs to prevent mother-to-child HIV transmission in two large Bangkok hospitals, 1999-2001. J Acquir Immune Defic Syndr 2005;38(2):208-212. [ Links ]
10. World Health Organization. Antiretroviral Drugs and the Prevention of Mother to Child Transmission of HIV Infection in Resource-Constrained Settings: Recommendations for Use. Geneva: WHO, 2004. [ Links ]
11. Doherty TM, McCoy D, Donohue S. Health system constraints to optimal coverage of the prevention of mother-to-child HIV transmission programme in South Africa: lessons from the implementation of the national pilot programme. Afr Health Sci 2005;5(3):213-218. [ Links ]
12. Naicker S, Plange-Rhule J, Tutt RC, Eastwood JB. Shortage of healthcare workers in developing countries - Africa. Ethn Dis 2009;19(1 Suppl 1):S1-60-4. [ Links ]
Accepted 26 November 2010.
Funding. This project was supported by the US Centre for Disease Control Program (South Africa).
Corresponding author: D Moodley (moodleyd1@ukzn.ac.za)














