Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
SAMJ: South African Medical Journal
versión On-line ISSN 2078-5135
versión impresa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.101 no.1 Pretoria ene. 2011
PART 2
GUIDELINES
Guideline for the management of chronic obstructive pulmonary disease - 2011 update
M S Abdool-GaffarI; A AmbaramII; G M AinslieIII; C T BolligerV; C FeldmanVII; L GeffenVIII; E M IrusenVI; J JoubertIX; U G LallooX; T T MabasoIX; K NyamandeXII; J O'BrienXIII; W OttoXIV; R RaineIV; G RichardsXV; C SmithXVI; D StickellsXVII; A VenterXVIII; S VisserIXX; M WongXX
IMB ChB, FCP. Pulmonologist in private practice, Amanzimtoti
IIMB BCh, Dippec(SA), FCP(SA), Cert Pulm(SA), FCCP. Department of Pulmonology and Critical Care, Inkosi Albert Luthuli Hospital, Durban
IIIMB ChB, FRCP. Respiratory Clinic, University of Cape Town and Groote Schuur Hospital
IVMB ChB, MMed (Med), FCP(SA). Respiratory Clinic, University of Cape Town and Groote Schuur Hospital
VMD, PhD, Bsc (Hons) (Epidem). Division of Pulmonology, Department of Medicine, Stellenbosch University and Tygerberg Academic Hospital, Tygerberg
VIMB ChB, FCP(SA), PhD. Division of Pulmonology, Department of Medicine, Stellenbosch University and Tygerberg Academic Hospital, Tygerberg
VIIMB BCh, DSc, PhD, FRCP, FCP(SA). Division of Pulmonology, Charlotte Maxeke Johannesburg Academic Hospital and University of the Witwatersrand
VIIIMB ChB. Doctor in private practice, Cape Town
IXFCP (SA), MD. Division of Pulmonology, Department of Medicine, Stellenbosch University
XMB ChB, MD, FCP(SA), DOH, FCCP, FRCP. Department of Pulmonology and Critical Care, Nelson R Mandela School of Medicine, University of KwaZulu-Natal and Inkosi Albert Luthuli Hospital, Durban
XIMB ChB. Doctor in private practice, Durban
XIIFCP MD. Department of Pulmonology and Critical Care, Nelson R Mandela School of Medicine, University of KwaZulu-Natal
XIIIMB ChB, FCP(SA). Pulmonologist in private practice, Cape Town
XIVMB ChB, MMed (Int). Department of Internal Medicine, University of the Free State, Bloemfontein
XVMB BCh, PhD, FCP(SA), FRCP. Division of Intensive Care, Charlotte Maxeke Johannesburg Academic Hospital and University of the Witwatersrand
XVIMB BCh, FCP(SA), MMed (Int Med), FCCP. Morningside Mediclinic, Sandton
XVIIMB ChB, Dip Obst(SA), FCP(SA). Pulmonologist in private practice, Port Elizabeth
XVIIIMB ChB, Dip pec(SA), DTMH (Wits), FCP(SA), cert pul(SA), Bsc (Hons) (Med). Pulmonologist in private practice, Pretoria
IXXMB ChB, M Med (Int Med), PhD (Imm). University of Pretoria and Steve Biko Academic Hospital
XXMB BCh, FCP(SA), FCCP, FRCP (Lond). Division of Pulmonology, Chris Hani Baragwanath Hospital and University of the Witwatersrand
ABSTRACT
OBJECTIVE: To revise the South African Guideline for the Management of Chronic Obstructive Pulmonary Disease (COPD) based on emerging research that has informed updated recommendations.
KEY POINTS:
1. Smoking is the major cause of COPD, but exposure to biomass fuels and tuberculosis are important additional factors.
2. Spirometry is essential for the diagnosis and staging of COPD.
3. COPD is either undiagnosed or diagnosed too late, so limiting the benefit of therapeutic interventions; performing spirometry in at-risk individuals will help to establish an early diagnosis.
4. Oral corticosteroids are no longer recommended for maintenance treatment of COPD.
5. A therapeutic trial of oral corticosteroids to distinguish corticosteroid responders from non-responders is no longer recommended.
6. Primary and secondary prevention are the most cost-effective strategies in COPD. Smoking cessation as well as avoidance of other forms of pollution can prevent disease in susceptible individuals and ameliorate progression. Bronchodilators are the mainstay of pharmacotherapy, relieving dyspnoea and improving quality of life.
7. Inhaled corticosteroids are recommended in patients with frequent exacerbations and have a synergistic effect with bronchodilators in improving lung function, quality of life and exacerbation frequency.
8. A cute exacerbations of COPD significantly affect morbidity, health care units and mortality.
9. Antibiotics are only indicated for purulent exacerbations of chronic bronchitis.
10. COPD patients should be encouraged to engage in an active lifestyle and participate in rehabilitation programmes.
OPTIONS:Treatment recommendations are based on the following: annual updates of the Global Obstructive Lung Disease (GOLD), initiative, that provide an evidence-based comprehensive review of management; independent evaluation of the level of evidence in support of some of the new treatment trends; and consideration of factors that influence COPD management in South Africa, including lung co-morbidity and drug availability and cost.
OUTCOME:Holistic management utilising pharmacological and nonpharmacological options are put in perspective.
EVIDENCE: Working groups of clinicians and clinical researchers following detailed literature review, particularly of studies performed in South Africa, and the GOLD guidelines.
BENEFITS, HARMS AND COSTS:The guideline pays particular attention to cost-effectiveness in South Africa, and promotes the initial use of less costly options. It promotes smoking cessation and selection of treatment based on objective evidence of benefit. It also rejects a nihilistic or punitive approach, even in those who are unable to break the smoking addiction.
RECOMMENDATIONS: These include primary and secondary prevention; early diagnosis, staging of severity, use of bronchodilators and other forms of treatment, rehabilitation, and treatment of complications. Advice is provided on the management of acute exacerbations and the approach to air travel, prescribing long-term oxygen and lung surgery including lung volume reduction surgery.
VALIDATION: The COPD Working Group comprised experienced pulmonologists representing all University departments in South Africa and some from private practice, and general practitioners. Most contributed to the development of the previous version of the South African guideline.
GUIDELINE SPONSOR:The meeting of the Working Group of the South African Thoracic Society was sponsored by an unrestricted educational grant from Boehringer Ingelheim and Glaxo-Smith- Kline.
Chronic obstructive pulmonary disease (COPD) is a significant cause of death and disability in both developed and developing countries. It is increasing in frequency and demands increasing utilisation of healthcare resources. Cigarette smoking remains the major cause of COPD but, in developing countries, biomass fuel smoke and tuberculosis are important additional causes.
This revised guideline is based on the following premises: (i) COPD is an inflammatory disorder, and (ii) while COPD is associated with partial reversibility of the airflow limitation using simple spirometry, modern treatment results in significant symptomatic improvement in quality of life (QOL) and morbidity.
1. Definition
COPD is a disease state resulting from an abnormal inflammatory response of the lungs to irritant particles and gases, with resultant progressive airflow limitation that is partially reversible. It is associated with lung hyperinflation and systemic effects. The pathological correlates are chronic bronchitis and emphysema.1,2
2. Epidemiology of COPD
COPD is one of the leading causes of morbidity and mortality in the world. It is estimated that there are currently over 280 million cases in the world, a significant proportion of whom are undiagnosed. The prevalence of COPD is projected to rise owing to increased exposure to risk factors and population ageing.3,4 A recent meta-analysis of 26 COPD studies in 28 countries reported a pooled prevalence estimate of 8.9%.5
The Burden of Obstructive Lung Disease (BOlD) study6 that investigated the prevalence of COPD in various countries showed that COPD prevalence varied considerably in different countries, with a range of 6 - 19% for stage >2 COPD (clinically significant disease) and 12 - 26% for stage >1 COPD. South Africa had the highest prevalence of stage >2 COPD (19% overall, 22% male and 17% female), which may be greater than the general South African prevalence because of the higher incidence of smoking, occupational dust exposure, indoor pollution and prior tuberculosis in the area studied. Unlike other chronic diseases, COPD mortality for both men and women is increasing. The Global Burden of Disease Study has projected that COPD, which was ranked 6th as the cause of death in 1990, will become the third leading cause of death by 2020.7,8
3. Risk factors
The prevalence of COPD is generally directly related to the prevalence of tobacco smoking, but other factors are also involved. The risk for COPD results from a host-environment interaction; the factors are shown in Table I.
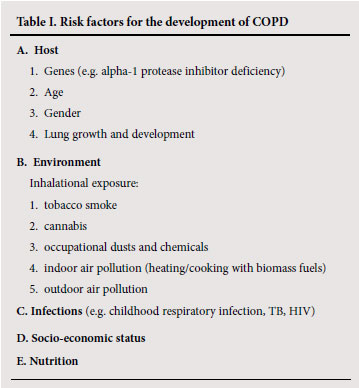
3.1 Host
Genetic
The genetic risk factor that is best documented is the hereditary deficiency of alpha-1 protease inhibitor.9 There is also an increased risk of airflow obstruction in the smoking siblings of patients with severe COPD, suggesting that other genetic factors are involved.10 Genes associated with COPD susceptibility in smokers are increasingly being identified.11
Age
COPD prevalence increases with age, but this probably relates more to the increased cumulative burden of exposure to risk factors (smoking, pollution, infection, etc.) than age per se.12,13
Gender
COPD prevalence and mortality is greater among men than women, and reflects previous smoking patterns. The prevalence is now equalising in developed countries. Women may be more susceptible than men to the effects of tobacco smoke.14
Lung growth and development
Poor lung growth owing to problems in utero and exposure during childhood is associated with airflow obstruction in adulthood.15,16
3.2 Environment
Inhalational exposure
Tobacco smoke
COPD is more common in smokers and ex-smokers than in nonsmokers. Cigarette smokers have more respiratory symptoms, lung function abnormalities, decline in FEV1, and COPD mortality than non-smokers. The starting age, total pack-years smoked and current smoking status are predictive of COPD morbidity and mortality.17 The annual decline of FEV1 in smokers varies from 55 -100 ml/ year v. About 30 ml/yr in non-smokers.18 However, not all smokers develop clinically significant COPD so genetic factors must modify risk.19 The oft-quoted figure of only 15% of smokers developing COPD is probably an under-estimate.20 Pipe and cigar smokers have greater COPD morbidity and mortality rates than non-smokers, although their rates are lower than those for cigarette smokers.
Passive or environmental tobacco smoke (ETs) may also contribute to COPD.21,22 Smoking during pregnancy may predispose to COPD by decreasing lung growth and development in the fetus.23
Cannabis
Smoking cannabis is associated with a dose-related airflow obstruction.24 One cannabis joint is equivalent to 2.5 -5 tobacco cigarettes.
Occupational dusts and chemicals
Occupational dust and chemical exposure is an independent cause of COPD.25 The large population-based NHANES III (National Health and Nutrition Examination Survey) utilising spirometry in almost 10 000 adults estimated that the fraction of COPD attributable to work was 19% overall and 31% among never-smokers.26
Indoor air pollution
The burning of biomass and fossil fuels (wood, animal dung, crop residues and coal) in open fires or poorly-functioning stoves in poorly-ventilated spaces leads to very high levels of indoor air pollution. Almost half the world's population, and 90% of rural families (including >20 million in South Africa), use biomass and coal as their main source of energy for cooking and heating.27,28 Several case-control and community-based studies have shown that this is associated with an increased risk for COPD in developing countries, especially among non-smoking women.29-31 It is estimated that 2 million women and children die globally in consequence each year.7
Outdoor air pollution
Air pollution, mainly from motor vehicle emissions in cities and biomass smoke (from bush and forest fires), is associated with loss of lung function.32-34
3.3 Infections
Childhood respiratory infections are associated with reduced lung function and increased respiratory symptoms in adulthood.35 several studies have shown that previous pulmonary tuberculosis may lead to COPD.36-39 HIV infection has been shown to accelerate the onset of smoking-related emphysema.40 In addition, viral and bacterial infections may contribute to the pathogenesis and progression of COPD.41
3.4 socioeconomic status
There is a higher prevalence of COPD among the lower socioeconomic strata,42 but this probably reflects increased exposure to smoking, indoor and outdoor air pollution, susceptibility to infections and poor nutrition.
3.5 Nutrition
The role of nutrition as an independent risk factor for the development of COPD is unclear. Severe malnutrition has been associated with emphysema.43
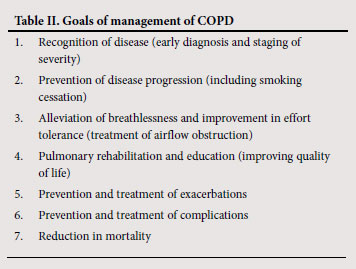
4. Aim of this guideline
The purpose of this guideline is to improve the care of patients with COPD at all levels of the health system in South Africa and to provide information that will assist in recognising and appropriately treating this common condition (Table II).44,45
5. Recognition of disease (early diagnosis and staging of severity)
The diagnosis of COPD should be considered in any patient with chronic progressive dyspnoea and/or chronic cough (with or without sputum production) with a smoking history of more than 10 pack-years and/or other risk factors for COPD.
Correct diagnosis, and in particular the differentiation of COPD from asthma, is important to ensure correct treatment. Clinical features that assist in the diagnosis of each condition are outlined in Table III. Early detection of COPD and effective smoking cessation interventions slow the decline in pulmonary function and may alter the natural history.

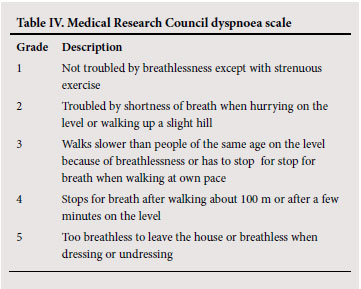
Clinical evaluation should include a history of exposure to tobacco smoking and other risk factors, an assessment of breathlessness (e.g. the MRC dyspnoea scale (Table IV)), the occurrence and severity of exacerbations, and co-morbid conditions that could complicate management. Physical examination may reveal signs of hyperinflation and airway obstruction but may be normal. Co-morbid conditions should also be sought.
5.1 spirometry
Spirometry is essential for the detection, assessment and management of patients with COPD. It must be performed by adequately trained persons using a spirometer of approved standard and quality that is calibrated frequently. Measurements used in the diagnosis of COPD are FEV1, FVC and FEV1/FVC% pre- and post-bronchodilator.
5.2 Detection of airflow obstruction
The presence of an FEV1/FVC ratio of less than 70% confirms the presence of airflow obstruction. The FEV1 is usually reduced (less than 80% of predicted value), and is used as a measure of severity. Most patients with symptomatic COPD have a reduced FEV1, but many patients with significantly reduced FEV1 have no symptoms.
5.3 Assessment of reversibility with short-acting bronchodilators
Spirometry should be performed before, and 20 minutes after, 4 puffs of a short-acting beta-2 agonist bronchodilator (e.g. Salbutamol, fenoterol or terbutaline). An improvement in FEV1 >12% from baseline and >200 ml indicates significant reversibility. This is compatible with asthma but is also seen in many patients with COPD. However, in general, the larger the improvement, the greater the likelihood that the diagnosis is asthma, especially with the clinical profile borne in mind (Table III).
5.4 Assessment of severity of COPD
To establish the severity of airflow obstruction, FEV1 is expressed as a percentage of predicted values. The European community for steel and coal(ECSC) (based on surveys performed in Europe)46 or the NHANES III reference equations47 are recommended for routine use in South Africa. Ethnic differences (amounting to approximately 12% lower values for FVC in Africans or African-Americans) have been demonstrated in some series, and a correction factor (multiplication of the spirometric value by 0.9) should be applied. Measurement of peak expiratory flow (PEF), while helpful in asthma, is not an appropriate test for diagnosing and evaluating the severity of COPD and does not distinguish obstructive from restrictive lung disease. The assessment of severity of COPD is based on spirometric measures and clinical indicators including severity of dyspnoea, functional impairment and 6-minute walking distance (6MWD).48 Grading of severity is used for prognostication and for selecting treatment. Details of the grades of severity are provided in Table V.
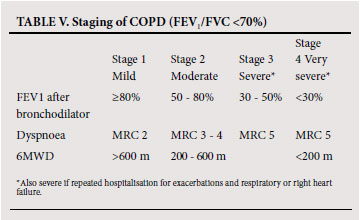
5.5 Monitoring disease progression
COPD progresses slowly over years. More rapid deterioration suggests an acute exacerbation or alternative diagnosis e.g. Asthma, cardiac failure and pneumothorax. Progression of the disease could be monitored using the parameters in Table V, but serial measurement of FEV1 alone is probably most practical.
5.6 Chest radiography
A chest radiograph may show evidence of hyperinflation but frequently appears normal. It is also useful for detecting additional disease.
6. Prevention of disease progression (including smoking cessation)
Avoidance of occupational and environmental pollution, including passive or 'side-stream' tobacco smoke exposure is important, particularly in susceptible persons: pregnant women, infants and children, persons with alpha-1 protease inhibitor deficiency, and those with COPD.
Smoking cessation is the only measure that has been shown to slow the progression of COPD, and is one of the most cost-effective interventions in health care.49 All smokers must be encouraged to stop smoking. Health care workers should be familiar with smoking cessation measures and promote these among their patients. 50 The advantages of cessation include: (i) slowing of the rate of decline of lung function (a small initial improvement in spirometry occurs in some), (ii) improved oxygen transport in blood, through reductions in carboxyhaemoglobin and blood viscosity, (iii) reduced tendency to thrombosis, (iv) improvements in appetite, body mass, muscle strength and exercise tolerance, (v) possible improved efficacy of some classes of drugs used in COPD, and (vi) delayed development of respiratory failure and cor pulmonale.
6.1 smoking cessation programmes
There are many smoking cessation methods. Many are behavioural in approach, but some are pharmacological and involve gradual weaning from nicotine via nicotine replacement, with or without mood modifiers to counteract the negative effects of withdrawal. Many interventions show impressive short-term results, but long-term abstinence (defined as cessation without relapse for 12 months) occurs in <30% of patients (for most methods, only 15 - 20%). Factors associated with poor success include heavy smoking and relapse within the first 2 weeks.
6.2 features that contribute to a successful programme
6.2.1 At an initial in-depth interview, discuss the patient's smoking habits and previous quit attempts. The 5 'A's' are an increasingly used memory aid for counseling smokers:
• 'Ask' do you smoke?
• 'Advise' to quit.
• 'Assess' the willingness to quit.
• 'Assist'( aid) the patient to quit (provide counseling and medication).
• 'Arrange' follow-up contact.
6.2.2 Cessation advised repeatedly by a doctor.
6.2.3. Abrupt cessation rather than gradual smoking reduction, with agreement on a quit date and provision of support for adherence to the commitment (by clinical visit or phone call). For recalcitrant smokers, reduction is possible and enhances subsequent cessation.
6.2.4. Reinforcement and follow-up.
6.2.5. There are three medications for smoking cessation: (i) nicotine replacement therapy (NRT) with 4 approved formulations: gum, patch, sublingual tablets and an oral spray, (ii) Bupropion, an antidepressant and (iii) varenicline, a newly developed partial nicotine agonist. These medications are mostly used as single treatments but, for more dependent smokers, combinations of more than 1 nrT or nrT with Bupropion have been shown to be effective.
7. Alleviation of breathlessness and improvement in effort tolerance (treatment of airflow obstruction)
7.1 Bronchodilators
Bronchodilators form the mainstay of the treatment of COPD, for which inhaled beta-2 agonists, inhaled anticholinergics and oral theophyllines are all effective. Individuals vary in their responsiveness to each, and combinations may have additive effects.51-53 Commence treatment in symptomatic patients with an inhaled short-acting bronchodilator on a PRN basis. Thereafter, increase treatment stepwise to include inhaled long-acting bronchodilators, slow-release theophylline and inhaled corticosteroids (ICS) in various combinations (see Fig. 1).
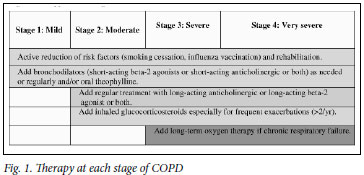
7.1.1 Inhaled short-acting beta-2 agonists
These agents have a rapid onset of action. Examples include salbutamol, fenoterol and terbutaline.
7.1.2 Inhaled long-acting beta-2 agonists
The long-acting beta-2 agonists (LABA) are salmeterol and formoterol. LABAs are more effective and more convenient than regular short-acting beta-2 agonists. Their use as monotherapy (without inhaled corticosteroids) appears to be safe (in contrast to asthma). LABAs improve symptoms, reduce exacerbations, reduce rescue therapy requirements and improve exercise capacity (Evidence A).54 Regular use does not result in loss of efficacy. Formoterol may also be used as reliever treatment because of its rapid onset of action.
7.1.3 Combination of LABA and ICS
A combination of LABA + ICS therapy:
• reduces the number of acute exacerbations in patients with moderate to severe COPD (Evidence A)
• improves FEV1 (Evidence B)
• improves health-related quality of life (Evidence B)
• may increase the risk of pneumonia (Evidence B).55,56
7.1.4 Inhaled anti-cholinergics
Anticholinergic agents are effective bronchodilators for COPD because the disease is associated with increased vagally mediated bronchoconstriction. Ipratropium bromide is a short-acting agent with a peak bronchodilator effect at approximately 40 minutes and between 4 - 6 hours duration of action. Tiotropium bromide is a long-acting anticholinergic with a slow onset of peak bronchodilation but a 24-hour duration of action facilitating once-daily administration. They are more effective than beta-2 agonists in many patients and have fewer side-effects (dry mouth and urinary retention). Ipratropium bromide is prescribed for as-needed use for symptom relief in COPD and can also be used 4 - 6-hourly as a maintenance bronchodilator agent. Tiotropium may be used as a first-line long-acting bronchodilator treatment in COPD or may be used in combination with LABAs because of their different mechanisms of action. Tiotropium has been shown to improve quality of life and reduce exacerbation rates and hospitalisation (Evidence A).57-60 It is available as a dry powder inhaler and requires an inspiratory flow rate of at least 30 l/min for optimal respiratory tract delivery.
7.1.5 Nebuliser treatment
This is an alternative for stage 3 and 4 patients with poor inhalation technique and/or acute dyspnoea.
• Nebulised ipratropium plus beta-2 agonist can be used up to 3 or more times daily.61,62 While there are distinct advantages, nebulisers tend to be overused.
• Patients requiring chronic nebuliser therapy should, where possible, have specialist assessment.
7.1.6 Theophyllines
Theophyllines have similar bronchodilator effects to beta-2 agonists. They improve quality of life, and the oral route of administration is an advantage for some patients.63 limitations include toxicity (particularly in the elderly), drug interactions and variable metabolism. The recommended dose of oral slow-release theophylline is 200 - 400 mg twice a day or 400 - 800 mg at night, and should not be exceeded without monitoring blood levels. Combination tablets containing theophylline and other bronchodilators or sedatives are not recommended.
7.1.7 Combination bronchodilator treatment
A combination of bronchodilator agents is recommended and has additive effects. The following combinations are effective:64,65
• SABA + ipratropium
• long-acting anti-cholinergic + SABA
• long-acting anti-cholinergic + LABA
• LABA + ipratropium.
Oral theophylline may be added to any combination of inhaled treatment.
7.2 Inhaled corticosteroids
Inhaled corticosteroids, particularly in combination with LABAs, have been shown to improve lung function, quality of life and reduce exacerbations.55,56 The best evidence for their efficacy is in patients with >3 exacerbations per year, especially in severe disease. Patients need to be carefully monitored for side-effects such as skin bruising and oral candidiasis.
7.3 Oral corticosteroids
Oral corticosteroids are no longer recommended for stable COPD.66 Low-dose oral corticosteroids only cause small improvements in lung function. Higher doses (>30 mg/d) are associated with improvements in lung function but also with major side-effects that include aggravation of blood pressure, hyperglycaemia, decreased adrenal function and osteoporosis. An oral corticosteroid trial is no longer utilised to determine corticosteroid-responsive subjects as such a phenotype cannot be distinguished.67 It is only recommended in instances where the clinical profile makes distinction between asthma and COPD difficult. A marked improvement in spirometry and symptoms following a corticosteroid trial would suggest asthma.
7.4 Newer phosphodiesterase inhibitors
Phosphodiesterase inhibitors, such as Roflumilast, have been shown to be effective in phase III clinical trials but are not yet registered for use in South Africa.
7.5 Mucolytics and mucokinetic agents
Expectoration of tenacious sputum is a distressing symptom. Unfortunately, mucolytics, mucokinetic drugs, cough syrups and acetylcysteine (oral and inhaled) have not been shown to be effective, and are not recommended.
7.6 Chest physiotherapy
An ineffective cough may be improved by instruction in the 'huff technique' of coughing and active cycle of breathing. Percussion and vibration therapy do not form part of routine management in stable patients. The physiotherapist has an important role in directing the conditioning (exercise) programme and in advising on breathing and coughing techniques.
7.7 Venesection
An increased haematocrit causes aggravation of cardiac failure, increased ventilation/perfusion abnormality and an increased incidence of thrombotic episodes. When the haematocrit is >0.55, therapeutic venesection should be considered; this can be repeated to maintain the haematocrit in the normal range.
7.8 Assessment of response to therapy
The FEV1 is useful as a diagnostic and prognostic tool and is also of value in monitoring disease progression. It is not as useful for assessing response to treatment. Other lung function measurements to monitor response include inspiratory capacity (IC), residual volume (RV) and functional residual capacity (FRC).68 The response to treatment can also be gauged by the improvement in dyspnoea, and the MRC dyspnoea scale (Table IV) is used to quantify the degree of dyspnoea. The 6-minute walking distance test can be used to assess response to therapy as well. (Appendix B).
8. Pulmonary rehabilitation and education (improving quality of life)
Rehabilitation is a team effort involving the doctor, physiotherapist and/or biokineticist. Conditions such as uncontrolled hypertension, and overt or silent cardiac ischaemia, have to be treated before inclusion in a programme.69-74 Details of a rehabilitation programme are described in Appendix D. Rehabilitation can be undertaken in resource-limited settings using supervised walking as a major component.
9. Prevention and treatment of acute exacerbations of COPD
9.1 Definition, natural history and diagnosis of exacerbations
An exacerbation is defined as an increase in symptoms of COPD above the usual day-to-day variation experienced by the patient, and necessitating a change in treatment. Exacerbations vary in both severity and frequency. They tend to become more common as COPD progresses. Exacerbations severely affect quality of life, may be associated with permanent worsening of COPD, account for a large percentage of the direct costs associated with the treatment of COPD, and increase mortality. The most common cause of exacerbations is tracheobronchial infections.
Acute exacerbations must be distinguished from other diseases and complications of COPD (including pneumonia, pneumothorax, congestive heart failure, arrhythmias and pulmonary embolism) that require alternative treatment. When exacerbations are associated with features of infection (pyrexia and purulent sputum), the presence of pneumonia and other forms of lower respiratory infection should be excluded using a chest radiograph. An approach to the antibiotic treatment of acute exacerbations of COPD is provided below (par. 9.3.3) and in Appendix C.
9.2 Prevention of exacerbations
The following measures have been shown to reduce the frequency and/or severity of COPD exacerbations (Evidence A):49,55-57,64,65,73
• smoking cessation
• prevention of respiratory infections (influenza and pneumococcal vaccination)
• bronchodilator treatment
• inhaled corticosteroids.
9.3 Management of exacerbations1,2,41,45
9.3.1 Bronchodilators
• Nebulisations with short-acting bronchodilators may be given 4-hourly (or as often as every 30 - 60 minutes or continuously in severe cases). Multiple actuations of an MDI delivered via a large (>500 ml) spacer device may be as effective as nebulisation.
• Should there be an inadequately sustained response to the above treatment, oral or intravenous theophyllines may be used.
9.3.2 Corticosteroids
Corticosteroids should preferably be given orally (Evidence A). A once-daily dose of 30 -40 mg prednisone is recommended to be continued for 7 - 10 days (Evidence C). Tapering is not required. An equivalent dose of an intravenous steroid may be given if the patient is unable to take oral medication.
9.3.3 Antibiotics (see Appendix C)
These should be prescribed when there is evidence of a severe exacerbation (as evidenced by the 3 cardinal symptoms of increased sputum volume, sputum purulence and increased dyspnoea) or in those who require ventilation (Evidence B). If the patient has 2 of the cardinal symptoms, of which one is increased sputum purulence, they should also receive antibiotics (Evidence C). Sputum Gram-stain may be of help by confirming the presence of relevant organisms, but is not an essential investigation. The organisms most commonly involved are Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis. With the increasing appearance of penicillin- and macrolide-resistant pneumococci, and betalactamase-mediated resistance of H. Influenzae and M. Catarrhalis, local sensitivity data should be considered when choosing an antibiotic. Macrolides should be avoided in areas where there are high levels of resistance. Alternatives are amoxicillin/clavulanate, cefuroxime, or fluoroquinolones. The oral route of administration is preferred except for severe illness. The duration of treatment should be 5 - 7 days (Evidence A).
9.3.4 Oxygen therapy
Oxygen should be started at 24% or 1 - 2 l/minute by nasal cannula. Increases should be gradual to avoid carbon dioxide narcosis. This should be guided by blood gas analysis or by the level of consciousness if blood gases are not available. The aim should be to maintain the saturation above 90%.
9.3.5 Physiotherapy to assist with clearance of secretions.
Postural drainage and chest percussion are of limited benefit and can aggravate fatigue and respiratory failure.
9.3.6 Indications for hospitalisation
• severe COPD
• any of the following features:
• sustained failure to improve on outpatient management
• inability to walk between rooms (where previously mobile)
• family and/or physician unable to manage the patient at home
• high-risk co-morbid condition, whether pulmonary (e.g. Pneumonia) or non-pulmonary
• prolonged progressive worsening of symptoms before emergency visit
• altered mentation
• worsening hypoxaemia and new or worsening hypercapnia
• new onset arrhythmia
• elderly or frail patient
• new or worsening right-sided cardiac failure unresponsive to outpatient management.
9.3.7 Indications for ICU admission
Pre-conditions for ICU admission:
• satisfactory functional status before the exacerbation (patient coped with activities of daily living); if not known, the patient should be given the benefit of the doubt
• possible need for mechanical ventilation i.e. PaO2 <6.7 kPa (50 mmHg) on room air, arterial blood pH <7.3, and confusion
• the presence of a reversible factor. Examples are infections, bronchospasm, oxygen-induced carbon dioxide narcosis, sedative administration, heart failure or other associated illnesses.
9.3.8 ventilatory support must be considered for the patient with one or more of the following features:
• exhaustion, confusion, coma
•pH <7.3 and declining (respiratory acidosis)
• respiratory or cardiac arrest
• inability to clear secretions.
Modalities of ventilatory support include invasive (mechanical ventilation) and non-invasive continuous positive airways pressure (CPAP) or BiPAP techniques. Patients considered suitable for non-invasive ventilation are those who are able to protect their airway and clear their airway secretions. Indications for non-invasive ventilation (NIV) include the following (all criteria should be met):
respiratory rate >30 breaths/minute
• pH <7.35
• pCO2 >6.7 kPa (>50 mmHg)
• paO2 <6.7 kPa (<50 mmHg) on room air.
Contra-indications to non-invasive ventilation include haemodynamic instability, decreased level of consciousness or poor co-operation, vomiting and excessive secretions. Appearance of any of these features in a patient undergoing NIV who still needs ventilation would be an indication for intubation and mechanical ventilation.
Non-invasive positive pressure ventilation in acute exacerbations has been shown to improve blood gases and pH, reduce in-hospital mortality, decrease the need for invasive mechanical ventilation, and decrease the length of hospital stay (Evidence A).
10. Prevention and treatment of complications
The main complications include right heart failure, severe exacerbations, pulmonary embolism, pneumothoraces, erythrocytosis and chronic respiratory failure.
10.1 The management of right heart failure
• Identify and treat the precipitating cause. In COPD, this might include an acute respiratory infection, worsening airflow obstruction (review bronchodilator and other treatment) or worsening hypoxaemia from additional factors such as a move to higher altitude or a thrombo-embolic event.
• Correction of hypoxaemia with long-term oxygen as indicated under long-term oxygen therapy (LTOT), see paragraph 6.3) (evidence D).
• Diuretics (e.g. hydrochlorothiazide 25 mg or equivalent). Avoid large decreases in preload which may precipitate hypotension and renal impairment.
• Digoxin must be avoided except in the presence of atrial fibrillation and/or left ventricular dysfunction/failure (Evidence C).
• Ace inhibitors and calcium antagonists are not indicated for the management of cor pulmonale or right ventricular failure (Evidence B).
• Prophylaxis with subcutaneous heparin to prevent deep vein thrombosis during periods of exacerbation or prolonged immobilisation (Evidence A).
• Chronic treatment with warfarin needs to be considered in COPD patients with atrial fibrillation or thrombo-embolic complications (Evidence A). There is no evidence of benefit when used routinely in patients with cor pulmonale secondary to COPD (Evidence C).
10.2 Pneumothorax
The development of a spontaneous pneumothorax must be considered when patients with stable COPD suddenly deteriorate, as patients with respiratory impairment tolerate even a small pneumothorax poorly.
10.3 Long-term oxygen therapy (LTOT)
The full benefit is only evident in patients with persistent hypoxaemia (with or without hypercapnia) in the stable phase of COPD. In such patients, it has been shown to reduce the complications of respiratory and right heart failure and to improve survival.
10.3.1 Indications for LTOT
• Stable, severe COPD (usually but not exclusively with an FEV1 <1.5 l and FEV1/FVC <70%) on optimal bronchodilator therapy.
The decision to prescribe LTOT should only be made once the patient has fully recovered from an acute exacerbation ( usually after 6 weeks).
• Arterial hypoxaemia (paO2 <7.3 kPa or 55 mmHg at sea-level) or oxygen saturation <90% at rest.
• Smoking cessation for >3 months.
Hypoxaemia must be confirmed by arterial blood gases performed while the patient is breathing room air. Following exacerbations of COPD, spirometry and PaO2 may continue to improve for up to 3 months. Therefore, these should ideally be checked in all patients on 2 occasions at least 1 month, and preferably 3 months, apart before prescribing LTOT. Continued smoking reduces the efficacy of treatment and is a contra-indication to oxygen therapy. Worsening hypercapnia caused by hypoventilation is an occasional complication of oxygen therapy in patients with severe COPD and hypercapnic respiratory failure. Regular follow-up by a suitably experienced physician and ready access to technical advice, either through a private contractor or a hospital department, must be available. Assessment of adherence is essential.
10.3.2 Oxygen prescription
Oxygen is administered by facemask or nasal cannula for a total of at least 16 hours per 24-hour day. A flow rate of 1 -2 l/minute is used, the rate being determined in each case by arterial blood gas determinations. Oximetry may be used for follow-up checks. Oxygen can be delivered by oxygen concentrators or by cylinders. Concentrators are more convenient and cost effective. Portable oxygen systems include bottled oxygen with flow-saving devices and portable concentrators. With these systems, patients do not have to be confined to home.
10.4 Palliative symptomatic oxygen therapy
Oxygen given for short periods to relieve breathlessness in hypoxic patients with COPD does not influence the natural progression of the disease and is therefore not routinely recommended. Patients in whom a fall in PaO2 occurs during exercise may improve their exercise capacity with supplemental oxygen. Patients who desaturate at night should be assessed for sleep apnoea and treated appropriately.
10.5 Additional considerations
10.5.1. Sleep in COPD
Sleep is associated with a decrease in arterial oxygen saturation (SaO2) in most individuals that is more marked in COPD. Significant night-time hypoxaemia cannot be predicted from measurement of daytime blood gas and pulmonary function tests, but if the daytime PaO2 is >8 kPa, nocturnal SaO2 need only be measured if unexplained respiratory failure, cor pulmonale or erythrocytosis are found. Referral for a full sleep study (polysomnography) and a specialised opinion should only be considered if sleep-disordered breathing is suspected (i.e. daytime hyper-somnolence and other symptoms of sleep deprivation, or a strong history of loud snoring with apnoeic events). Long-term oxygen therapy, prescribed for daytime hypoxaemia, must be used during sleep.
10.5.2. Surgery for COPD
Surgical techniques that can improve lung function and symptoms of COPD include:
• bullectomy
• lung volume reduction surgery
• lung transplantation.
Bullectomy
This may be indicated to decompress adjacent lung parenchyma if there are large localised bullae. This can be performed thoracoscopically, and may reduce dyspnoea and improve lung function (Evidence C).
Lung volume reduction surgery (LVRS)
In this procedure, parts of the lung are resected to reduce hyperinflation. It is an alternative to lung transplantation in patients with severe inhomogeneous emphysema who remain symptomatic despite optimal medical therapy. The FEV1 improves by about 10% with larger improvements in exercise tolerance, dyspnoea and quality of life.
The ideal candidate should be carefully selected by a multidisciplinary team including the general practitioner, pulmonologist and thoracic surgeon. Guidelines include a FEV1 20 -35% of that predicted, diffusion capacity for carbon monoxide >20% predicted, hyperinflation with inhomogeneous disease and limited co-morbidities. In experienced units, survival after 4 years is 54% v. 39.7% for medical therapy in patients with mainly upper lobe emphysema and low exercise capacity. LVRS remains an expensive palliative surgical procedure and is recommended only in carefully selected patients. new techniques to effect LVR using valves or biological substances are being investigated.
Lung transplantation
This approach may be used in patients with diffuse severe emphysema, but access to transplantation is severely limited in South Africa. Its effect on survival after 2 years remains controversial. General guidelines recommend that the patient must be <65 years old without any other medical condition that could shorten predicted survival. Additional criteria for referral include FEV1 <35% predicted, paO2 <7.3 -8.0 kPa, pacO2 >6.7 kPa and secondary pulmonary hypertension.
10.5.3 surgery in COPD
Post-operative pulmonary complications constitute a significant cause of morbidity and mortality. The frequency varies from 2 -70%. The complications are atelectasis, infection (bronchitis and pneumonia), respiratory failure necessitating prolonged mechanical ventilation, exacerbation of COPD, bronchospasm and pulmonary embolism. Stratification of risk for pulmonary complications entails patient-related as well as procedure-related risk factors. Preoperative evaluation depends strongly on taking a thorough history and clinical examination. It is reasonable to perform a preoperative chest radiograph in patients with cardiopulmonary disease and those >50 years undergoing high-risk surgery. Pre-operative preparation, optimisation of airway function (bronchodilators, steroids, antibiotics and chest physiotherapy), smoking cessation, weight reduction where applicable, delay of elective surgery if chest infection is present, and patient education on deep breathing exercises, coughing, pain control and incentive spirometry are recommended. Post-operative measures include early mobilisation and ambulation, prophylactic lung expansion manoeuvres, adequate analgesia and prophylaxis against thrombosis.
10.5.4 spacer devices and delivery methods
The use of spacer devices may improve delivery of medication to the lower airways in patients with poor inhaler technique and poor lung function. Some patients may achieve better results with dry powder devices. It is vital to ensure that the individual uses the inhaler device correctly.
10.5.5 Air travel
COPD patients who travel by commercial aircraft put themselves at risk for a range of complications. Respiratory incidents comprise 9 - 10% of airborne emergencies. Therefore the need to travel needs to be balanced against the potential risks. Potential travelers with COPD should have stable disease (no recent exacerbations, hospitalisations or acute illnesses). Co-morbid disease increases the risks of in-flight emergencies i.e. Ischaemic heart disease, congestive cardiac failure and cor pulmonale (56% of all in-flight emergencies). Potential problems include:
• hypoxaemia at altitude: stable COPD patients with an oxygen saturation (SaO2) of 95% preflight desaturate to about 86% at a cabin altitude of 2 165 m. This worsens to 78% with activity.
• worsening of existing hypoxia at ground level
• pulmonary hypertension
• worsening discomfort and dyspnoea at altitude
• cardiac dysrhythmias
• risk of contracting a respiratory infection
• pneumothorax from large bullae.
The most practical evaluation includes room air SaO2 measurements pre-flight. If the SaO2 is less than 92%, further assessment is necessary. Available tests are :
• 50 m walk test. Those who desaturate will require supplemental O2.
• hypoxic inhalation test. Patients breathe 15% FiO2 at sea level. Those with significant desaturation will need supplemental O2.
• hypobaric chamber simulating in-flight conditions .Their limited availability precludes widespread use in assessing patients. The in-flight requirement would be 2 l/min higher than home use, or 2 - 4 l/minute if the assessment indicates the need for O2.
Acknowledgements
Several components of this guideline represent an unchanged standard of care and are based on the previous edition of the South African Guideline of which professor E D Bateman was the chief author; his contribution is gratefully acknowledged.
References
1. Bateman ED, Feldman C, O'Brien J, Joubert JR. Guideline for the Management of COPD: 2004 revision. S Afr Med J 2004;94:559-575. [ Links ]
2. Rodriguez-Roisin R, Rabe KF, Anzueto A, et al. Global Initiative for Chronic Obstructive Lung Disease(GOLD). Global strategy for diagnosis, management, and prevention of chronic obstructive pulmonary disease. Bethesda, MD: NHLBI, 2008. http://www.goldcopd.com (accessed 4 May 2009). [ Links ]
3. Lopez AD, Shibuya K, Rao C, et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J 2006;27:397-412. [ Links ]
4. Celli BR. Update on the management of COPD. Chest 2008;133:1451-1462. [ Links ]
5. Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS , Mannino DM. Global burden of COPD: systematic review and meta-analysis. Eur Respir J 2006;28:523-532. [ Links ]
6. Buist AS , McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (The BOLD Study): a population-based prevalence study. Lancet 2007;370:741-750. [ Links ]
7. Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet 1997;349:1498-1504. [ Links ]
8. Jemal A, Ward E, Hao Y, Thun M. Trends in the leading causes of death in the United States, 1970-2002. JAMA 2005;294:1255-1259. [ Links ]
9. Stoller JK, Aboussouan LS. Alpha1-antitrypsin deficiency. Lancet 2005;365:2225-2236. [ Links ]
10. McCloskey SC, Patel BD, Hinchliffe SJ, et al. Siblings of patients with severe chronic obstructive pulmonary disease have a significant risk of airflow obstruction. Am J Respir Crit Care Med 2001;164:1419-1424. [ Links ]
11. Ammous Z, Hackett NR, Butler MW, et al. Variability in small airway epithelial gene expression among normal smokers. Chest 2008;133:1344-1353. [ Links ]
12. Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS , Mannino DM. Global burden of COPD: systematic review and meta-analysis. Eur Respir J 2006;28:523-532. [ Links ]
13. Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC. Chronic obstructive pulmonary disease surveillance - United States, 1971-2000. MMWR Surveill Summ 2002;51:1-16. [ Links ]
14. Silverman EK, Weiss ST, Drazen JM, et al. Gender-related differences in severe, early-onset chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000;162:2152-2158. [ Links ]
15. Barker DJ, Godfrey KM, Fall C, Osmond C, Winter PD, Shaheen SO. Relation of birth weight and childhood respiratory infection to adult lung function and death from chronic obstructive airways disease. Br Med J 1991;303:671-675. [ Links ]
16. Lawlor DA, Ebrahim S, Davey, Smith G. Association of birth weight with adult lung function: findings from the British Women's Heart and Health Study and a meta-analysis. Thorax 2005;60:851-858. [ Links ]
17. Burrows B, Knudson RJ, Cline MG, Lebowitz MD. Quantitative relationships between cigarette smoking and ventilatory function. Am Rev Respir Dis 1977;115:195-205. [ Links ]
18. Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J 1977;1:1645-1648. [ Links ]
19. Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of Lung Health Study participants after 11 years. Am J Respir Crit Care Med 2002;166:675-679. [ Links ]
20. Rennard S, Vestbo J. COPD: the dangerous underestimate of 15%. Lancet 2006;367:1216-1219. [ Links ]
21. Eisner MD, Balmes J, Katz BP, Trupin L, Yelin E, Blanc P. Lifetime environmental tobacco smoke exposure and the risk of chronic obstructive pulmonary disease. Environ Health Perspect 2005;4:7-15. [ Links ]
22. Dayal HH, Khuder S, Sharrar R, Trieff N. Passive smoking in obstructive respiratory disease in an industrialized urban population. Environ Res 1994;65:161-171. [ Links ]
23. Tager IB, Ngo L, Hanrahan JP. Maternal smoking during pregnancy. Effects on lung function during the first 18 months of life. Am J Respir Crit Care Med 1995;152:77-83. [ Links ]
24. Tashkin DP. Does cannabis use predispose to chronic airflow obstruction? Eur Respir J 2010;35:3-5. [ Links ]
25. Becklake MR. Occupational exposures: evidence for a causal association with chronic obstructive pulmonary disease. Am Rev Respir Dis 1989;140:85-91. [ Links ]
26. Hnizdo E, Sullivan PA , Bang KM, Wagner G. Association between chronic obstructive pulmonary disease and employment by industry and occupation in the US population: a study of data from the Third National Health and Nutrition Examination Survey. Am J Epidemiol 2002;156:738-746. [ Links ]
27. Jaakkola MS, Jaakkola JK. Biomass fuels and health. Am J Resp Crit Care Med 2006;174:851-852. [ Links ]
28. Ezzati M. Indoor air pollution and health in developing countries. Lancet 2005;366:104-106. [ Links ]
29. Regalado J, Perez-Padilla R, Sansores R, et al. The effect of biomass burning on respiratory symptoms and lung function in rural Mexican women. Am J Resp Crit Care Med 2006;174:901-905. [ Links ]
30. Liu S, Zhou Y, Wang X, et al. Biomass fuels are the probable risk factor for chronic obstructive pulmonary disease in rural South China. Thorax 2007;62:889-897. [ Links ]
31. Dennis RJ, Maldonado D, Norman S, Baena E, Martinez G. Woodsmoke exposure and risk for obstructive airways disease among women. Chest 1996;109:115-1192. [ Links ]
32. Oroczo-Levi M, Garcia-Aymerich J, Villar J, Ramirez-Sarmiento A, Anto JM, Gea J. Wood smoke exposure and risk of chronic obstructive pulmonary disease. Eur Respir J 2006; 27:542-546. [ Links ]
33. Sezer H, Akkurt I, Guler N, Marakoglu K, Berk S. A case-control study on the effect of exposure to different substances on the development of COPD. Ann Epidemiol 2006;16:59-62. [ Links ]
34. Abbey DE, Burchette RJ, Knutsen SF, McDonnell WF, Lebowitz MD, Enright PL . Long-term particulate and other air pollutants and lung function in non-smokers. Am J Respir Crit Care Med 1998;158:289-298. [ Links ]
35. Shaheen SO, Barker DJ, Shiell AW , Crocker FJ, Wield GA, Holgate ST. The relationship between pneumonia in early childhood and impaired lung function in late adult life. Am J Respir Crit Care Med 1994;149:616-619. [ Links ]
36. Pasipanodya JG, Miller TL, Vecino M, et al. Pulmonary impairment after tuberculosis. Chest 2007;131:1817-1824. [ Links ]
37. Hnzido E, Singh T, Churchyard G. Chronic pulmonary function impairment by initial and recurrent pulmonary tuberculosis following treatment. Thorax 2000;55:32-38. [ Links ]
38. Long R, Maycher B, Dhar A, et al. Pulmonary tuberculosis treated with directly observed therapy: serial changes in lung structure and function. Chest 1998;113:933-943. [ Links ]
39. de Valliere S, Barker RD. Residual lung damage after completion of treatment for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2004;8:767-771. [ Links ]
40. Diaz PT, King MA, Pacht ER, et al. Increased susceptibility to pulmonary emphysema among HIVseropositive smokers. Ann Intern Med 2000;132:369-372. [ Links ]
41. Seemungal T, Harper-Owen R, Bhowmik A, et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;164:1618-1623. [ Links ]
42. Prescott E, Lange P, Vestbo J. Socioeconomic status, lung function and admission to hospital for COPD: results from the Copenhagen City Heart Study. Eur Respir J 1999;13:1109-1114. [ Links ]
43. Coxson HO, Chan IH, Mayo JR, Hlynsky J, Nakano Y, Birmingham CL. Early emphysema in patients with anorexia nervosa. Am J Respir Crit Care Med 2004;170:748-752. [ Links ]
44. Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004; 23: 932-946. [ Links ]
45. National Institute for Clinical Excellence (NICE ). Chronic obstructive pulmonary disease. National clinical guideline on management of chronic obstructive pulmonary disease in adults in primary and secondary care. Thorax 2004;59 Suppl 1:1-232. [ Links ]
46. Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report working party standardization of lung function tests, european community for steel and coal. Official statement of the European Respiratory Society. Eur Respir J Suppl 1993;16:5. [ Links ]
47. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999;159:179-187. [ Links ]
48. Celli BR, Cote CG, Marin JM, et al The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004;350:1005-1012. [ Links ]
49. Tonnesen. Smoking cessation in patients with respiratory diseases: a high priority, integral component of therapy. Eur Respir J 2007;29:390-417. [ Links ]
50. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. Public Health Service report. Am J Prev Med 2008;35:158-176. [ Links ]
51. Ikeda A, Nishimura K, Koyama H, Izumi T. Bronchodilating effects of combined therapy with clinical dosages of ipratropium bromide and salbutamol for stable COPD; comparison with ipratropium bromide alone. Chest 1995;107(2):401-405. [ Links ]
52. Friedman M, Serby CW, Menjoge SS, et al. Pharmacoeconomic evaluation of combination of ipratropium plus albuterol compared with ipratropium alone and albuterol alone in COPD. Chest 1999;115:635. [ Links ]
53. Belman MJ, Botnick WC , Shin JW. Inhaled bronchodilators reduce dynamic hyperinflation during exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1996;153:967-975. [ Links ]
54. Mahler DA, Donohue JF, Barbee RA , et al. Efficacy of salmeterol xinafoate in the treatment of COPD. Chest 1999;115:957-965. [ Links ]
55. Anzueto A, Ferguson GT, Feldman G, et al. Effect of fluticasone propionate/salmeterol (250/50) on COPD exacerbations and impact on patient outcomes. COPD 2009;6:320-329. [ Links ]
56. Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007;356:775-789. [ Links ]
57. V ncken W, van Noord JA, Greefhorst AP, et al. Improved health outcomes in patients with COPD during 1 yr's treatment with tiotropium. Eur Respir J 2002;19:209-216. [ Links ]
58. O'Donnell DE, Fluge T, Gerken F, et al. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J 2004;23:832. [ Links ]
59. Anzueto, A, Tashkin, D, Menjoge, S. One-year analysis of longitudinal changes in spirometry in patients with COPD receiving tiotropium. Pulm Pharmacol Ther 2005;18:75. [ Links ]
60. Van Noord JA, Aumann JL, Janssens E, et al. Comparison of tiotropium once daily, formoterol twice daily and both combined once daily in patients with COPD. Eur Respir J 2005;26:214. [ Links ]
61. Moayyedi P, Congleton J, Page RL, Pearson SB, Muers MF. Comparison of nebulised salbutamol and ipratropium bromide with salbutamol alone in the treatment of chronic obstructive pulmonary disease. Thorax 1995;50:834-837. [ Links ]
62. Gross N. Tashkin D, Miller R, et al. Inhalation by nebulization of albuterol-ipratropium combination [Dey combination] is superior to either agent in the treatment of chronic obstructive pulmonary disease. Respiration 1998;65:354. [ Links ]
63. Ram,FS, Jones, PW, Castro, AA, et al. Oral theophylline for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2002; CD003902. [ Links ]
64. Aaron SD, Vandemheen KL, Ferguson D, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med 2007;146:545. [ Links ]
65. Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543-1554. [ Links ]
66. Walters JAE, Walter EH, Wood-Baker R. Oral Corticosteroids for stable COPD. Cochrane Database of Systematic Reviews 2005. CD 005374. [ Links ]
67. Burge S, Calverly PMA, Jones PW, Spencer S, Anderson JA. Prednisolone response in patients with COPD: results from ISOLDE. Thorax 2003;58:654-658. [ Links ]
68. Marin JM, Carrizo SJ, Gascon M, Sanchez A, Gallego B, Celli BR. Inspiratory capacity, dynamic hyperinflation, breathlessness and exercise performance during the 6-minute-walk test in chronic obstructive pulmonary disease Am J Resp. Crit Care Med 2001;163:1395-1399. [ Links ]
69. Mohsenifar Z, Lee SM, Diaz P, et al. Single-breath diffusing capacity of the lung for carbon monoxide. A predictor of PaO2, maximum work rate and walking distance in patients with emphysema Chest 2003;123:1394-1400. [ Links ]
70. De Klerk D. An adapted rehabilitation program for a cross section of South African chronic obstructive pulmonary disease patients. PhD thesis,Faculty of Sport Science, Stellenbosch University, 2008. [ Links ]
71. Casaburi R, Kukafka D, Cooper CB, Witek TJ Jr, Kesten S. Improvement of exercise tolerance with the combination of tiotropium and pulmonary rehabilitation in patients with COPD. Chest 2005;127: 809-817. [ Links ]
72. Peters MM, Webb KA , O'Donnell DE. Combined physiological effects of bronchodilators and hyperoxia on exertional dyspnoea in normoxic COPD. Thorax 2006;61:559-567. [ Links ]
73. Garcia-Aymerich, Lange P, Benet M, Schinohr P, Antó IM. Regular physical activity reduces hospital admissions and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax 2006;61:772-778. [ Links ]
74. Morgan MD. Preventing hospital admissions for COPD: role of physical activity. Thorax 2003;58:95-96. [ Links ]
75. ATS statement. Guidelines for the six-minute walk test Am J Resp Crit Care Med 2002;166:111-117. [ Links ]
Accepted 14 September 2010.
Corresponding author: M Abdool Gaffar (docsabs@gmail.com)
Appendix A
Process of guideline revision
In 2008, the council of the South African Thoracic society resolved to revise the 2004 COPD guideline and appointed a convenor ( Dr Abdool-Gaffar) and editorial committee, selected a review date and agreed on the process of revision. Membership of the working Group comprised most members of the previous working group and general practitioners. The editorial committee identified areas of the guideline that required revision and commissioned members of the working group to prepare reviews of new data in these areas, with particular reference to the content of the latest version of the Global Obstructive lung Disease Guideline. The workshop was held in august 2008. A draft revision document, based on the consensus obtained at the workshop, was compiled by the editorial board, and circulated to the guideline committee for further comment in 2009. These comments were considered by the editorial committee, and the final draft was then completed and approved by the council of the South African Thoracic society by postal vote in May 2010.
The 6-minute walk test (6MWT)75
The 6MwT is a composite and repeatable test of lung, heart and muscle function. Measurement of specific exercise-induced outcomes are an extremely important adjunct to clinical and lung function evaluation. The 6MwT does not correlate well with FEV1 but relates to the degree of exercise-induced hyperinflation of COPD patients, and is used extensively as a prognostic indicator for patients with COPD, interstitial lung disease or pulmonary hypertension. It provides better insight into the degree of exercise impairment compared with single evaluations such as spirometry or an ECG.
The 6MwT has to be conducted under supervision of a trained medical or paramedical person who can recognise untoward exercise-induced symptoms. Prior to the test, patients have to be evaluated to rule out uncontrolled hypertension, ischaemic heart disease, arrhythmias and heart failure that may complicate the assessment or endanger the patient. These conditions need adequate treatment before the 6MwT can be conducted. Patients with lower limb arthritis or claudication and those who are mentally challenged or old and infirm will not perform the test adequately. It can be conducted in any facility that has the necessary resuscitation equipment and a straight, flat surface of 30 meters or a treadmill. Prior to the test, patients need to be counseled to motivate them to achieve the greatest distance. They also need to be informed that they may rest during the test but that it will be recorded as part of their 6MwT walk time. All COPD patients need to use their bronchodilators before attempting the 6MwT, and they should not be exercised during an infective episode or if they are in the immediate recovery phase of an exacerbation.
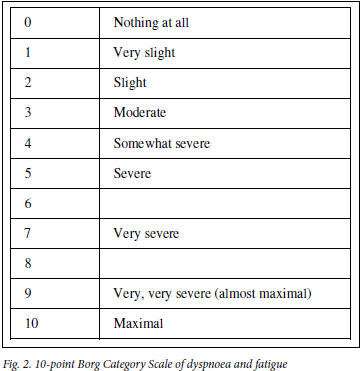
A baseline ECG is done and should be repeated after the first effort to record any asymptomatic exercise-induced ischaemic changes. The oxygen saturation of blood should be measured before and after the test. Determination of pulse rate, blood pressure and respiratory rate along with the blood oxygen saturation level should be recorded before and after the test. A level in the latter <90% or a decline >5% will identify those patients who will require oxygen during the test. Patients' opinions as to their degree of exhaustion need to be recorded on a Borg scale card (Fig. 2) which provides a semi-quantitative estimation of fatigue and dyspnoea. Recovery time should be noted by measuring the respiratory and heart rates at 3 and 5 minutes after the test. Repeatability of the 6MWD (distance) of 10% should be achieved. In this respect, a second or, if required, even a third 6MwT should be conducted after periods of rest of up to 20 minutes. The 6MwT should be discontinued in patients who complain of chest pain, intolerable dyspnoea or leg cramps, or appear to be staggering or have an ashen or pale appearance. A mean value for males of 464 metres and 430 metres for females is the standard for a healthy american population. A post-intervention improvement of 54 meters above the baseline 6MWD has been found to represent a clinically meaningful improvement in COPD patients who undergo rehabilitation.
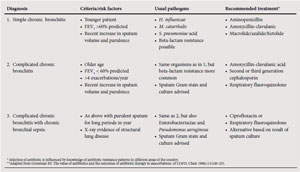
Antibiotic use in exacerbations of COPD
Chronic bronchitis, with attendant mucociliary dysfunction and mucus hypersecretion, is the major pathological component that is responsible for the risk of bacterial colonisation and acute infective exacerbations of COPD.
Recommendations for the antibiotic management of acute exacerbations of COPD based on a classification of bronchitis
Pulmonary rehabilitation
Rehabilitation is a team effort in which the doctor, a physiotherapist or biokineticist motivate patients to achieve an exercise-orientated lifestyle. All COPD patients who are dyspnoeic, regardless of their measured FEV1, qualify for the rehabilitation process. Factors such as exercise-induced hypoxia and exercise-induced hyperinflation in subsets of COPD patients aggravate the muscle weakness and enhance the level of dyspnoea.
A thorough pre-rehabilitation evaluation needs to be done by an appropriately experienced doctor. Three aspects should be considered:
• The first is to identify individuals who should be temporarily precluded from the rehabilitation programme. Conditions such as uncontrolled hypertension and overt or silent cardiac ischaemia that may cause heart failure have to be treated before inclusion. Additionally, those who have acute lower respiratory tract infections or arrhythmias will first require treatment for their immediate illness.
• Secondly, the evaluation should include a baseline measurement of Cardiac and lung function such as spirometry, dyspnoea assessment as well as a baseline 6MwT. These measurements will serve as points of reference for comparison during and after the rehabilitation process.
• A third important aspect of the evaluation is explaining the rehabilitation process, emphasising the goals that need to be achieved and general motivation to achieve the best outcome.
Rehabilitation improves exercise capacity, reduces perceived dyspnoea during exertion, enhances the health-related quality of life and reduces the number of hospitalisations and days in hospital. Patients should be prepared for at least 25 rehabilitation sessions of one hour each under the guidance of a trained staff member. The sessions should be conducted at a tempo of 2 -3 per week. Goal-setting and motivation should be maintained throughout to retain the advantages achieved during the programme. The programme should include balanced endurance and muscle strengthening exercises with emphasis on the thigh, shoulder and respiratory muscles. The rehabilitation process complements and enhances quality of life, dyspnoea improvement and exercise tolerance to a greater extent than pharmacological treatment alone. Patients who initially desaturate during the pre-rehabilitation evaluation should not be excluded. If their O2 saturation drops below 90%, the rehabilitation programme should be conducted with the patient on oxygen.
In a South African study,70 individuals who underwent rehabilitation predominantly in the open air under professional supervision improved their 6MwT, quality of life estimation and degree of dyspnoea to the same extent as a group of patients who had been rehabilitated in a fully equipped gymnasium. The importance of maintaining a long-term exercise programme for COPD patients to lower their risk for hospital admission and death has been established. In this context, the post-rehabilitation evaluation conducted by the referring physician, during which the 6MwT and dyspnoea calculation is repeated and compared with the pre-rehabilitation values, can be extremely rewarding for patients. Regular enquiry and motivation by the doctor during the long-term follow-up is required to keep patients from slipping back into an inactive lifestyle.