Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
SAMJ: South African Medical Journal
versión On-line ISSN 2078-5135
versión impresa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.100 no.9 Pretoria sep. 2010
FORUM
ISSUES IN RESEARCH
Prolonged deferral of antiretroviral therapy in the SAPIT trial: did we need a clinical trial to tell us that this would increase mortality?
Andrew Boulle; Polly Clayden; Karen Cohen; Ted Cohen; Francesca Conradie; Krista Dong; Nathan Geffen; Ashraf Grimwood; Rocio Hurtado; Christopher Kenyon; Stephen Lawn; Gary Maartens; Graeme Meintjes; Marc Mendelson; Megan Murray; Molebogeng Rangaka; Ian Sanne; David Spencer; Jantjie Taljaard; Ebrahim Variava; W D Francois Venter; Douglas Wilson
Tuberculosis is the major cause of morbidity and mortality in HIVinfected patients in sub-Saharan Africa. HIV infection is often first diagnosed following a diagnosis of tuberculosis, with many patients needing antiretroviral therapy (ART). Starting ART in HIV-infected patients with tuberculosis (TB) may be associated with complications, including side-effects from co-administration of multiple drugs with many overlapping toxicities, reductions in concentrations of certain antiretroviral drugs following the induction of metabolising enzymes and drug transporters by rifampicin, and paradoxical deterioration due to the immune reconstitution inflammatory syndrome (IRIS). Furthermore, the high pill burden of co-treatment could reduce adherence, resulting in poor treatment outcomes for both diseases. These potential harms must be weighed against the high mortality rates in patients with HIV-associated tuberculosis who do not receive ART, especially those with low CD4 counts. The optimal time to initiate ART in patients with tuberculosis is an important research question, and randomised controlled trials are addressing this issue.
Interim results of the South African Starting Antiretroviral Therapy at Three Points in Tuberculosis Therapy (SAPIT) study have been published in the New England Journal of Medicine.1 The SAPIT investigators are the first group to publish controlled data on when to initiate ART in patients with TB. Patients with sputum smear-positive pulmonary TB and CD4 counts below 500 cells/µl were randomised to start ART in one of three phases of TB treatment: within 4 weeks of starting TB therapy; within 4 weeks after the completion of the intensive phase of TB therapy (2 or 3 months of 4 or 5 anti-TB drugs for patients with new or retreatment TB, respectively); or within 4 weeks after the completion of TB treatment (6 or 8 months for patients with new or retreatment TB, respectively). Recruitment into the latter group (the sequential arm) was stopped prematurely by the Data Safety Monitoring Board (DSMB) when it was found that mortality was significantly higher in this group compared with the groups that started ART during TB therapy. Mortality largely occurred in patients with CD4 counts below 200 cells/µl. The groups starting ART during and after the intensive phase of TB treatment completed enrolment, and results are still pending.
When the SAPIT study was done we believe that their trial design was appropriate to investigate the optimal treatment strategy for the sub-set of patients with CD4 cell counts in the range 200 -500 cells/µl. In contrast, however, we believe that it was predictable that patients with advanced disease (CD4 counts <200 cells/µl) would experience higher mortality if ART was deferred for a long period and that these patients should not have been enrolled. These views were expressed in a letter and a short commentary, and on blogs by bioethicists and clinicians.2-5 The SAPIT investigators and several international researchers have defended the study design.3-7 However, none of these forums allowed for a thorough exploration of the ethical issues and the evidence related to outcomes in patients with HIV-associated TB that was available when the SAPIT study was conducted.
We explore in greater detail the key ethical issues raised by the SAPIT study and the relevant clinical evidence available to the researchers at the time the study started. The latter point is important, as people who have defended the SAPIT study have accused critics of employing current evidence rather than evidence that was available when the study commenced in mid-2005.6,7 We do not question the integrity of the researchers who conducted the study, several of whom are highly respected for their considerable contributions to HIV research in Africa. Responsibility for ethical study design and protection of patients participating in research is shared between investigators, ethics committees, regulatory bodies, and data and safety monitoring boards. We believe that people involved in research involving human subjects at all these levels can learn important lessons from the issues raised by the SAPIT study.
Clinical evidence
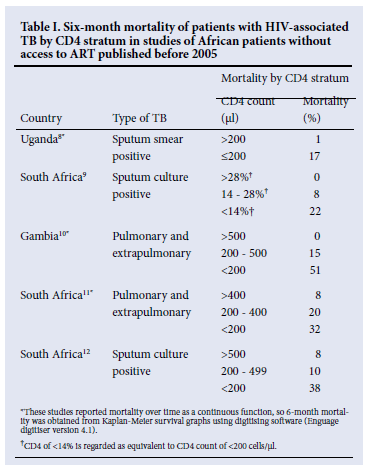
African studies, including three from South Africa, have reported mortality rates of patients with HIV-associated TB not on ART stratified by the degree of immune suppression.8-12 We extracted data on mortality at 6 months from these studies, as this is the duration of TB therapy for patients with first episodes of TB (Table I). All studies show dramatic increases in mortality as CD4 declines, with 6-month mortality rates of 17% to 51% in patients with CD4 counts of 200 cells/µl and below. Three studies showed that mortality in patients with CD4 counts below 200 cells/µl was similar in patients with and without TB.8,10,11 Patients in the SAPIT study were all sputum smear positive and received prophylactic co-trimoxazole, both of which are associated with lower mortality.13,14 Nevertheless it is clear that mortality is high during the course of TB therapy in patients with advanced HIV disease without ART.
The most important potential cause of harm resulting from starting ART with TB therapy is the development of IRIS, which occurs more commonly with earlier initiation of ART. At the time the SAPIT study started a systematic review of IRIS related to mycobacterial infections was published - the authors described life-threatening IRIS reactions in patients with TB, but failed to find a single fatal case reported in the literature.15 A more recent meta-analysis estimated the incidence of paradoxical TB IRIS to be 15%, with mortality among these of 3%.16 Extrapolating from these data, an estimated 0.45% of patients with HIV-associated TB starting ART die as a result of paradoxical TB IRIS.
Apart from IRIS, the complications of co-treatment with ART and TB drugs have not been associated with increased mortality. Rifampicin, a key component of TB therapy, induces many drugmetabolising enzymes and drug transporters, which can lead to reductions in concentrations of the non-nucleoside reverse transcriptase inhibitors (NNRTIs), used in first-line ART regimens, and may impair responses to ART. At the time of the SAPIT study it was recognised that efavirenz was the preferred NNRTI to be used with rifampicin-based TB therapy, with limited but good outcome data, and this was recommended in both the national and World Health Organization (WHO) guidelines implemented at the time.17,18 Efavirenz was used in the SAPIT study. Data on overlapping drug toxicities of ART and TB drugs were limited at the time of the SAPIT study, but we found no evidence to suggest that these were associated with an increased risk of mortality.
Two retrospective studies, published before the SAPIT study started, showed reductions in mortality and new opportunistic diseases in patients starting ART during TB therapy compared with those who deferred ART.19,20 Another study found that outcomes were similar for patients without TB starting ART and for those starting ART during TB treatment.21
In summary, the evidence available to the SAPIT investigators when the study started showed that HIV-infected patients with CD4 counts below 200 cells/µl have high mortality during TB treatment, that TB IRIS is associated with a low risk of mortality (with none reported when the SAPIT study started), that effective ART regimens were available that could be taken with TB treatment, and that ART reduced mortality when commenced during TB treatment.
The cited studies to obtain this evidence are observational. Randomised controlled trials provide a better level of evidence than observational studies, as the randomisation process should ensure that groups are well matched - this is the main rationale for the SAPIT study. However, numerous randomised controlled trials have been done on ART regimens over the past two decades. The monotherapy antiretroviral era was the last time that no therapy or placebo was used for patients with advanced HIV disease. Once reductions in mortality had been shown with monotherapy, subsequent trials compared monotherapy with dual therapy, and then dual therapy with triple therapy, which resulted in dramatic reductions in mortality and is the current standard of ART care.22
The key point is that ART is directed at suppressing HIV replication, resulting in gradual recovery of the immune system. The benefit of ART in reducing the frequency of opportunistic diseases and mortality follows from this immune recovery. ART has no direct effect on any opportunistic diseases such as TB. Any study of the timing of initiation of ART for HIV-associated complications must be mindful of the extensive evidence base that exists for ART.
Ethical issues
Equipoise
For randomisation to occur, published data should show conflicting or ambiguous results resulting in substantial clinical uncertainty, so that at the time of randomisation there is an equal (balanced) risk of individual patients experiencing either harm or benefit in any of the study arms.23 This situation is called equipoise. The investigators of the SAPIT study have claimed that there was equipoise, with the principal investigator stating 'When you put them on ARVs, they died. When you didn't, they died. We were at sea. As discussed, '3 we found evidence that ART significantly reduced mortality in patients with HIV-associated TB and could not find fatal cases of TB IRIS reported in the literature before the SAPIT study started. The other complications of TB/HIV co-treatment are unlikely to cause significant increases in mortality. In our view, none of the concerns about TB/HIV co-treatment overrules the high risk of untreated HIV infection in patients with advanced disease.
The SAPIT investigators also argued7 that the 2003 WHO ART guidelines18 support their view that there was equipoise, citing the following text: 'The optimal time to initiate ART in patients with TB is not known.'
The WHO guidelines go on to state (italics our emphasis): 'Case-fatality rates in many patients with TB during the first 2 months of TB treatment are high, particularly when they present with advanced HIV disease, and ART in this setting might be lifesaving. On the other hand, pill burden, drug-to-drug interaction, potential toxicity and immune reconstitution syndrome should be kept in mind when deciding on the best time to begin treatment ... Pending current studies, WHO recommends that ART in patients with CD4 cell counts below 200/mm3 be started between two weeks and two months after the start of TB therapy, when the patient has stabilized on this therapy. This provisional recommendation is meant to encourage rapid initiation of therapy in patients among whom there may be a high mortality rate. However, deferring the start of ART may be reasonable in a variety of clinical scenarios. For example, in patients with higher CD4 cell counts the commencement of ART may be delayed until after the induction phase of TB therapy is completed in order to simplify the management of treatment.'
We agree with these views in the 2003 WHO ART guidelines. For patients with CD4 counts below 200 cells/µl there was equipoise for comparing different time-points for starting ART during the intensive phase of TB treatment (or possibly shortly after the intensive phase, as with one group in the SAPIT trial). For patients with CD4 counts above 200 cells/µl there may have been equipoise for starting ART shortly after TB treatment.
Standard of care
Ethical principles for medical research involving human subjects in the World Medical Association's Declaration of Helsinki24 recommend that 'the best current' intervention should be provided as the standard of care to patients in studies, including those in the control group, except when there is no intervention (which is not the case for HIV infection) or 'where for compelling and scientifically sound methodological reasons the use of placebo is necessary to determine the efficacy or safety of an intervention and the patients who receive placebo or no treatment will not be subject to any risk of serious or irreversible harm'. The latter point is intended primarily for conditions with minor morbidity, and they go on to state: 'Extreme care must be taken to avoid abuse of this option.' The Declaration of Helsinki is internationally recommended for medical research and all research projects in South Africa should abide by its principles.
Insisting on the 'best current' standard of care has been criticised, as standards of care may differ in high-and low-income countries, and important research could be stifled in developing countries.25 The WHO 2003 ART guidelines18 differed substantially from guidelines for high-income countries at the time (e.g. standardised regimens, lower CD4 threshold for starting ART), but set the standard of care for resource-limited settings and allowed for rapid scale-up of ART globally.
The 2004 South African ART guidelines,17 largely based on the WHO guidelines, recommended the following for starting ART in patients with TB: 'If the patient has a history of WHO stage IV illness, and/or a CD4 count of less than 200 cells/mm3 , complete 2 months of TB therapy before commencing ART. If the patient has a CD4 count of less than 50 cells/mm3 , or other serious HIV-related illnesses, make sure that the patient is tolerating TB treatment before initiating ART.'
Consequently, care for patients in the group who delayed ART until after TB treatment in the SAPIT study with CD4 counts below 200 cells/µl was below the standard of care. On ethical considerations of the standard of care, Lie et al. state: 'Participants should not be denied any treatments with significant benefits that they would ordinarily receive. In this sense, research participants should be no worse off than they would be if they did not participate in the trial.'25 Patients in Durban with HIV-associated TB and CD4 counts below 200 cells/ µl would have been better off had they been given the prescribed standard of care in the South African health system at the time rather than enrolled into the sequential arm of the SAPIT study.
The SAPIT investigators argue that patient safety in the deferred ART group was ensured by the protocol allowing clinicians to start ART at any point if the patients deteriorated.7 We do not accept that this ensures patient safety, as patients with low CD4 counts may appear relatively well one day and then deteriorate rapidly with life-threatening conditions such as bacterial infections or pneumocystis pneumonia. It may be too late at this point to intervene with ART, which works by suppressing viral replication, resulting in improvement of CD4 count and function. This takes months to achieve in most patients.
Suggestions for a way forward
The issues we have raised have implications for the future conduct of clinical trials and patient safety in South Africa. Research ethics committees face a daunting task, with the explosion of medical knowledge making it difficult for them to find appropriate independent reviewers for research protocols from their own institutions. One option would be the development of a South African registry of reviewers, which would need to respect the intellectual property of the research protocols. Investigators should provide detailed information on the current standard of care, and any study offering less than this standard should undergo wide consultation before the study is passed. Research ethics committees should consider requesting reviews from other institutions, locally and internationally, more frequently than at present, especially with interventional studies. It is not mandatory for studies to be passed by research ethics committees/institutional review boards of the institutions of all investigators, except under certain circumstances (e.g. if the funding comes from a foreign institution and the research is conducted in South Africa, then the foreign investigator institutions should also review the study). However, it is desirable that studies be reviewed by all these institutions, as this increases the chances that problems can be identified. Finally, the ethical training of investigators and the capacity of ethics committees in South Africa should be improved.
1. Abdool Karim SS, Naidoo K, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med 2010; 362: 697-706. [ Links ]
2. Wilson D, Meintjes G. Timing of antiretroviral drugs during tuberculosis therapy. N Engl J Med 2010; 362: 2137. [ Links ]
3. Cohen J. Bioethicists assail a celebrated TB/HIV treatment trial. Science 2010; 328: 799-801. [ Links ]
4. Philpott S, Schuklenk U. A study that should not have been done. 5 May 2010. http://www.thehastingscenter.org/Bioethicsforum/Post.aspx?id=4626& blogid=140#ixzz0qRT08kb2 (accessed 9 June 2010). [ Links ]
5. Sonnabend J. An unethical study? 18 May 2010. http://blogs.poz.com/joseph/archives/2010/05/an_unethical_study.html (accessed 5 July 2010). [ Links ]
6. Cullinan K. TB trial splits HIV community. http://www.health-e.org.za/news/article.php?uid=20032822 (accessed 9 June 2010). [ Links ]
7. Abdool Karim SS, El-Sadr W, Friedland G. Timing of antiretroviral drugs during tuberculosis therapy. N Engl J Med 2010; 362: 2137. [ Links ]
8. Whalen CC, Nsubuga P, Okwera A, et al. Impact of pulmonary tuberculosis on survival of HIV-infected adults: a prospective epidemiologic study in Uganda. AIDS 2000; 14: 1219-1228. [ Links ]
9. Murray J, Sonnenberg P, Shearer SC, Godfrey-Faussett P. Human immunodeficiency virus and the outcome of treatment for new and recurrent pulmonary tuberculosis in African patients. Am J Respir Crit Care Med. 1999; 159: 733-740. [ Links ]
10. Van der Sande MA, van der Loeff MF, Bennett RC, et al. Incidence of tuberculosis and survival after its diagnosis in patients infected with HIV-1 and HIV-2. AIDS 2004; 18: 1933-1941. [ Links ]
11. Badri M, Ehrlich R, Pulerwitz T, Wood R, Maartens G. Association between tuberculosis and HIV disease progression in a high tuberculosis prevalence area. Int J Tuberc Lung Dis 2001; 5: 225-232. [ Links ]
12. Churchyard GJ, Kleinschmidt I, Corbett EL, Murray J, Smit J, De Cock KM. Factors associated with an increased case-fatality rate in HIV-infected and noninfected South African gold miners with pulmonary tuberculosis. Int J Tuberc Lung Dis 2000; 4: 705-712. [ Links ]
13. Connolly C, Davies GR, Wilkinson D. Impact of the human immunodeficiency virus epidemic on mortality among adults with tuberculosis in rural South Africa, 1991-1995. Int J Tuberc Lung Dis 1998; 2: 919-925. [ Links ]
14. Wiktor SZ, Sassan-Morokro M, Grant AD, et al. Efficacy of trimethoprimsulphamethoxazole prophylaxis to decrease morbidity and mortality in HIV1-infected patients with tuberculosis in Abidjan, Côte d'Ivoire: a randomised controlled trial. Lancet 1999; 353: 1469-1475. [ Links ]
15. Lawn SD, Bekker LG, Miller RF. Immune reconstitution disease associated with mycobacterial infections in HIV-infected individuals receiving antiretrovirals. Lancet Infect Dis 2005; 5: 361-373. [ Links ]
16. Müller M, Wandel S, Colebunders R, Attia S, Furrer H, Egger M. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect Dis 2010; 10: 251-261. [ Links ]
17. Department of Health. National Antiretroviral Treatment Guidelines, 1st edition, 2004. http://www.hst.org.za/uploads/files/sa_ART_Guidelines1.pdf (accessed 2 March 2010). [ Links ]
18. World Health Organization. Scaling Up Antiretroviral Therapy in Resource-limited Settings: Treatment Guidelines for a Public Health Approach. Geneva: World Health Organization, 2003. http://whqlibdoc.who.int/publications/2004/9241591552.pdf (accessed 14 June 2010). [ Links ]
19. Dean GL, Edwards SG, Ives NJ, et al. Treatment of tuberculosis in HIV-infected persons in the era of highly active antiretroviral therapy. AIDS 2002; 16: 75-83. [ Links ]
20. Dheda K, Lampe FC, Johnson MA, Lipman MC. Outcome of HIV-associated tuberculosis in the era of highly active antiretroviral therapy. J Infect Dis 2004; 190: 1670-1676. [ Links ]
21. Hung CC, Chen MY, Hsiao CF, Hsieh SM, Sheng WH, Chang SC. Improved outcomes of HIV-1-infected adults with tuberculosis in the era of highly active antiretroviral therapy. AIDS 2003; 17: 2615-2622. [ Links ]
22. Jordan R, Gold L, Cummins C, Hyde C. Systematic review and meta-analysis of evidence for increasing numbers of drugs in antiretroviral combination therapy. BMJ 2002; 324: 757. [ Links ]
23. Ashcroft R. Equipoise, knowledge and ethics in clinical research and practice. Bioethics 1999; 13: 314-326. [ Links ]
24. World Medical Association Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects. http://www.wma.net/en/30publications/10policies/b3/index.html (accessed 2 March 2010). [ Links ]
25. Lie RK, Emanuel E, Grady C, Wendler D. The standard of care debate: the Declaration of Helsinki versus the international consensus opinion. J Med Ethics 2004; 30: 190-193. [ Links ]
Andrew Boulle, School of Public Health and Family Medicine, University of Cape Town;
Polly Clayden, HIV I-Base;
Karen Cohen, Division of Clinical Pharmacology, Department of Medicine, University of Cape Town;
Ted Cohen, Division of Global Health Equity, Brigham and Women's Hospital, Harvard Medical School, USA, and Department of Epidemiology, Harvard School of Public Health;
Francesca Conradie, Clinical HIV Research Unit, University of the Witwatersrand, Johannesburg;
Krista Dong, iTEACH/Edendale Hospital, and Massachusetts General Hospital, Harvard Medical School;
Nathan Geffen, Treatment Action Campaign;
Ashraf Grim wood, Kheth'Impilo;
Rocio Hurtado, Division of Infectious Diseases, Department of Medicine, Massachusetts General Hospital, Harvard Medical School;
Christopher Kenyon, Department of Medicine, University of Cape Town;
Stephen Lawn, Desmond Tutu HIV Centre, Institute for Infectious Disease and Molecular Medicine, University of Cape Town, and Clinical Research Unit, Department of Infec tious and Tropical Diseases, London School of Hygiene and Tropical Medicine;
Gary Maartens, Division of Clinical Pharmacology, De partment of Medicine, University of Cape Town;
Graeme Meintjes, Institute of Infectious Diseases and Molecular Medicine, University of Cape Town;
Marc Mendelson, Division of Infectious Diseases and HIV Medicine, Department of Medicine, University of Cape Town;
Megan Murray, Division of Global Health Equity, Brigham and Women's Hospital, Harvard Medical School, and Department of Ep idemiology, Harvard School of Public Health;
Molebogeng Rangaka, Institute of Infectious Diseases and Molecular Medicine, University of Cape Town;
Ian Sanne, Department of Medicine, University of the Witwatersrand;
David Spencer, Toga Molecular Laboratory, Edenvale, Johannesburg;
Jantjie Taljaard, Division of Infectious Dis eases, Department of Medicine, Tygerberg Academic Hospital and Stellenbosch University;
Ebrahim Variava, Department of Medicine, Klerksdorp Tshepong Hospital Complex;
W D Francois Venter, Re productive Health and HIV Research Unit, University of the Wit watersrand;
Douglas Wilson, Department of Medicine, Edendale Hospital and University of KwaZulu-Natal.
Corresponding author: G Maartens (gary.maartens@uct.ac.za)














