Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.100 n.8 Pretoria Aug. 2010
ORIGINAL ARTICLES
The microbiology of acute complicated bacterial sinusitis at the University of the Witwatersrand
Ian Paul Olwoch
MB ChB, MMed (Anaesth), MMed (Otorhinolaryngol). Department of Otorhinolaryngology, Head and Neck Surgery, University of the Witwatersrand, Johannesburg
ABSTRACT
DESIGN: A retrospective chart review study at two referral hospitals identified 226 consecutive surgical patients with acute complicated sinusitis.
SUBJECTS: One hundred and fifty-nine male and 67 female patients, with a mean age of 16.5 (standard deviation 0.7) years, underwent external fronto-ethmoidectomy with maxillary sinus washout and 13 had a concurrent craniotomy.
RESULTS: A total of 233 micro-organisms were isolated from 163 patients (72.1%), and 63 (27.9%) were culture negative. Positive isolates included Streptococcus milleri (18.5%), Staphylococcus aureus (12.4%), β-haemolytic streptococci (10.8%), coagulase-negative staphylococci (8.6%), Haemophilus influenzae (8.6%) and the anaerobes, Peptostreptococcus (6.4%) and Prevotella (4.7%) species. The prevalences of S. pneumoniae (2.6%), methicillin-resistant S. aureus (MRSA) (1.3%) and Moraxella catarrhalis (0.4%) were low. Polymicrobial disease was present in 56 patients (34.4%). There was a significant difference between the two hospitals in the prevalences of some bacteria (p<0.05).
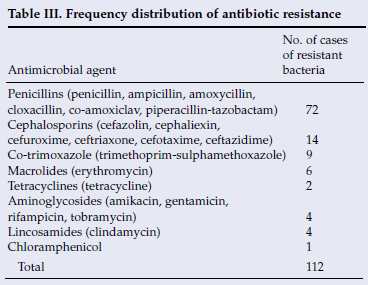
Antibiotic resistance was highest towards the penicillins (64.3%) and cephalosporins (12.5%). Effective empiric treatment was achieved with metronidazole and a choice of amoxicillin-clavulanate or ampicillin plus cloxacillin or penicillin plus chloramphenicol.
CONCLUSION: The polymicrobial nature and severity of complicated sinusitis warrants a de-escalation approach to antimicrobial therapy. The combination of β-lactamase- resistant penicillins and metronidazole is a reasonable choice for initial empiric antibacterial therapy.
Selection of drugs for empirical antibiotic therapy in patients with acute complicated sinusitis should be supported by knowledge of the local prevalence and antimicrobial susceptibilities of bacteria isolated from patients.
The microbiology of bacterial sinusitis has been studied extensively. The most common pathogens cited are aerobes and facultative anaerobes that include Streptococcus pneumoniae, Haemophilus influenzae, Staphylococcus aureus, Moraxella catarrhalis, Klebsiella pneumoniae, Pseudomonas aeruginosa and S. milleri.1- 9 Anaerobic organisms, isolated from as many as one-third of patients, include Propionibacterium acnes and Peptostreptococcus, Prevotella, Enterobacter, Bacteroides and Fusobacterium species.9,10 Despite the abundance of published studies there are very few that report on the microbiology of acute complicated sinusitis.3-8,11,12
Complicated sinusitis is associated with debilitating and potentially life-threatening orbital, intracranial, bone and soft-tissue complications; its treatment is both a medical and a surgical emergency.3,5,6,11,13,14 At the University of the Witwatersrand, patients with complicated sinusitis are referred to the Department of Otorhinolaryngology where they usually present with any combination of sinus involvement (maxillary, ethmoid, frontal and sphenoid) and one or more associated complications.3,5,6,11,13,14 A routine clinical assessment and computed tomography scan diagnosis is made before any surgery. Prompt medical treatment, with or without surgery, is then commenced. This entails initial empirical use of antibiotics before laboratory identification of the offending pathogens. As in other centres, observed differences in the local prevalence of bacterial pathogens and their antibiotic susceptibilities will influence the choice of drugs used at the start of treatment.1,3,4,6,7
The aims of this study were to determine the bacteriology and antibiotic susceptibility in isolates from patients with complicated sinusitis at the University of the Witwatersrand and to discuss an empirical basis for the initial choice of antibiotic drugs at this institution.
Materials and methods
A 5-year (January 2002 - December 2006) retrospective hospital chart review of patients with acute complicated sinusitis who underwent external fronto-ethmoidectomy (or ethmoidectomy) with maxillary sinus washout was performed. Institutional approval was obtained for the study (Protocol No. M070203). Patients were identified from the registers of the operating theatre, surgical wards and radiology department in each of two university hospitals situated 30 km apart: Johannesburg (JH) and Chris Hani Baragwanath (CHB) hospitals. The database of the resident National Health Laboratory Service (NHLS) was searched for corresponding records of microbiological investigations performed on sinus specimens derived at the time of surgery.
Sinus aspirate and tissue specimens were collected intra operatively from affected sinuses, and standard aseptic conditions of collection were presumed to have been applied. The specimens were then transported to the laboratory, usually within 2 hours of collection. Sinus aspirates and ground tissues were inoculated onto 5% horse blood agar, MacConkey agar, chocolate agar and thioglycolate broth for aerobic bacteria and incubated at 35ºC for 24 - 48 hours. Specimens for anaerobic culture were obtained directly from the surgical samples and indirectly as a subculture from thioglycate broth. Anaerobic specimens were inoculated onto 10% horse blood agar and amikacin blood agar and incubated at 35ºC for 24 - 48 hours. Direct Gram staining and microscopy were first performed after 24 hours of incubation and then repeated as necessary at 48, 72 and 96 hours.
The predominant pathogens isolated in the microbiology cultures were used for statistical analysis. The following variables were analysed: (i) gender; (ii) age (paediatric 0 - 15 years or adult >15 years); (iii) presence (or absence) of intracranial complications; (iv) number of different species (monomicrobial or polymicrobial) found within a single specimen; and (v) hospital from which the sample was obtained.
Only cases with positive sinus microbiological cultures were included in the evaluation. Statistical analysis was performed using Student's t-test and the chi-square (χ2) test. A p-value of <0.05 was regarded as significant.
Results
A total of 301 surgical patients were identified; 75 were excluded from the study owing to an absence of laboratory records (42), sinus histology reports without corresponding microbiology reports (29), incomplete microbiology reports (3) and rejected specimens (1).
Microbiology culture results were obtained for 226 patients, 159 males and 67 females (mean age 16.5 (standard deviation (SD) 0.7) years). All underwent external frontoethmoidectomy with maxillary sinus washout and 13 had a concurrent craniotomy. One hundred and sixty-three specimens (72.1%) were culture positive and 63 (27.9%) culture negative. A total of 233 micro-organisms were isolated from the culture-positive specimens (Table I). Aerobes and facultative anaerobes accounted for 85.5% of the isolates, anaerobes for 13.3% and fungi for 1.2%. The most frequently isolated aerobic micro-organisms were S. milleri (18.5%), S. aureus (12.4%), β-haemolytic streptococci (10.3%), coagulase-negative staphylococci (8.6%) and H. influenzae (8.6%). S. pneumoniae (2.6%) and M. catarrhalis (0.4%) were not common pathogens. The prevalence of methicillin-resistant S. aureus (MRSA) was very low (1.3%). The anaerobes isolated were mainly Peptostreptococcus (6.4%) and Prevotella (4.7%) spp.
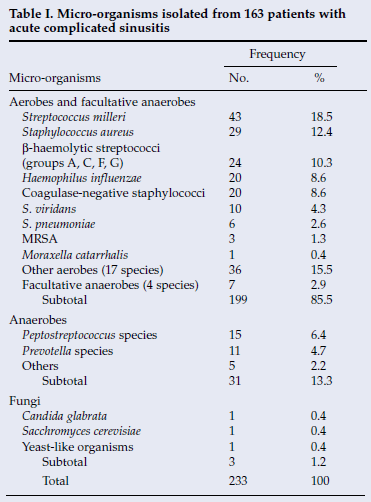
Comparison by gender (Table II). Males (71.8%) were affected more often than females (28.2%), with a male/female ratio of 2.7:1. Of the 31 patients with intracranial complications, 29 males and 3 females were affected (data not shown). No significant differences in the occurrence of bacterial species were observed within the gender study group.
Comparison of paediatric and adult patients (Table II). There were 70 adults (mean age 24.2 (SD 1.3) years) and 93 children (mean age 10.8 (SD 0.3) years) with culture-positive specimens. H. influenzae was isolated more frequently from the paediatric group (p<0.05) and coagulase-negative staphylococci mainly from adults (p<0.05).
Comparison by presence or absence of intracranial complications (Table II). Patients with and without intracranial complications were affected by the same species of bacteria and to a similar extent. S. milleri was the most common microorganism and was isolated from 48.4% and 21.2% of patients with and without complications, respectively.
Comparison of monomicrobial and polymicrobial specimens (data not shown). Isolates from 65.6% of the culture-positive specimens were monomicrobial, while 34.4% were polymicrobial. The mean number of species recovered per specimen was 1.4 (range 1 - 4). One specimen contained four species, namely S. milleri, S. viridans, H. influenzae and Photobacter species. A significantly higher (p<0.05) percentage of aerobic and facultative anaerobic bacteria (95.3%) were isolated from monomicrobial specimens compared with polymicrobial specimens (75.4%). Anaerobes were isolated in 4.7% and 24.6% of monomicrobial and polymicrobial specimens, respectively (p<0.05). Peptostreptococcus and Prevotella spp. were isolated exclusively from polymicrobial specimens.
Comparison of specimens from JH and CHB (Table II). A similar study population of patients was found at each of the two hospitals. Sixty-nine culture-positive patients (42.3%) aged 3 - 48 years (mean 18.4 (SD 1.2) years) were sourced from JH and 94 patients, aged 4 - 74 years (mean 15.3 (SD 1.0) years), from CHB. Eighty-seven and 146 micro-organisms were isolated from JH and CHB, respectively. S. milleri was the most common isolate at CHB (28.1%), where it occurred more frequently (p<0.05) than at JH (2.3%). Beta-haemolytic streptococci were the most common micro-organisms found at JH (20.7%) and were a more frequent (p<0.05) isolate than at CHB (4.8%). Coagulase-negative staphylococci were also a more frequent (p<0.05) pathogen at JH (14.9%) than at CHB (4.8%). There was no significant difference between the two hospitals in the reported frequencies of S. aureus and Peptostreptococcus and Prevotella species.
Antibiotic susceptibility (data not shown). Records were obtained for antibiotic susceptibility for 78 microorganisms; however, susceptibility tests were not consistently reported for all common antibiotics, and significance levels were therefore not tested. Penicillin, ampicillin and erythromycin were highly effective against S. milleri, β-haemolytic streptococci, S. pneumoniae and streptococcal species. S. aureus and coagulase-negative staphylococci were highly susceptible to cloxacillin and resistant to penicillin. Coagulase-negative staphylococci showed no resistance to either ampicillin or erythromycin. H. influenzae was susceptible to ampicillin in 7 of 9 cases. MRSA was susceptible to vancomycin.
Antibiotic resistance (Table III). One hundred and twelve instances of antibiotic resistance were recorded. The highest occurrence of resistance was to the penicillins (64.3%), cephalosporins (12.5%) and co-trimoxazole (8%). There were 4 reports of resistance of anaerobic bacteria to clindamycin, and no susceptibility tests to metronidazole were documented.
Discussion
Initial empiric antibiotic therapy in patients with acute complicated sinusitis should ideally be guided by the knowledge of the most prevalent causative pathogens and their antimicrobial resistance patterns. The prevalence of pathogenic bacteria and antimicrobial resistance patterns may vary widely between different centres located within the same geographical region.1,4-6 It is therefore important for medical practitioners to be aware of the existing trends in the communities in which they practise. In medical literature the aerobic species cited as being among the most prevalent include S. aureus,3,6,7,11-13 H. influenzae,1,2,6,9 S. milleri,4,5 S. pneumoniae,2,9,12 S. viridans3,6 and M. catarrhalis.2,9 The most frequently reported anaerobes include Peptostreptococcus, Prevotella, Fusobacterium, Propionibacterium and Bacteroides.5,9-11
In this study 226 cultures were recovered, predominantly from young male patients. Of these 163 (72.1%) were culture positive and yielded a total of 233 isolates. Negative culture rates are a measure of prior antibiotic consumption, specimen handling and laboratory standards, and may vary widely from 0% to above 50%.6,12 In this study, the negative culture rate of 27.9% is comparable to that encountered in similar studies.2,4,11
Recent publications mention S. milleri as being the most common pathogen in patients with complicated sinusitis.4,5,8 Whereas this is in agreement with the overall findings of this study, it was true of CHB (28.1%) but not of JH (2.3%). The notable absence of S. milleri as seen at JH is also reported by other authors.3,6,7,11-13
Lancefield group A, C, F and G β-haemolytic streptococci (10.8%) were isolated at both hospitals. Group C, with 11 isolates, had the highest count, followed by groups A and F, then finally by group G (Table I). As a group they were the most common isolate at JH (20.7%) but not at CHB (4.8%). Few studies on complicated sinusitis make specific mention of β-haemolytic streptococci as a pathogen.1,7,15 In one recent study the authors present what they consider to be the first reported case of the isolation of group C streptococcus, in complicated sinusitis, from a child with an orbital sub-periosteal abscess.15
In this study S. aureus (12.4%) was the second most common isolate. This is in keeping with the universal acceptance of this organism as a major pathogen.3-8,11,14,15
H. influenzae (8.6%) and coagulase-negative staphylococci (8.6%) ranked joint third. H. influenzae occurred more frequently in children than in adults (Table II). Previous studies cite wide variations, of between 2.8% and 26%, in the incidence of positive isolates of H. influenzae in complicated sinusitis.1,2,4-6,12 Equivalent studies attach no importance to H. influenzae as a pathogen.3,8,11,13
Coagulase-negative staphylococci have long been regarded as non-pathogenic, but in a recent study their role as important pathogens is recognised.6 In this study coagulase-negative staphylococci occurred more frequently in adults than in children (Table II).
S. pneumoniae (2.6%) was infrequent and was not isolated from any patient with intracranial complications in this series. Reports from several smaller studies quote higher isolation rates, ranging from 7.7% to 11.9%.1,2,4,6,8,12 On the other hand, total absence of S. pneumoniae is also reported.7
Studies have shown M. catarrhalis to be a frequent isolate in cultures from patients with acute sinusitis.2,9 This study identified only one isolate of M. catarrhalis (0.4%), which supports the consensus that this organism is not a major pathogen in complicated sinusitis.1,3- 6,11-14
Anaerobic bacteria may account for as much as one-third of all isolates.5,6 In this study they accounted for 13.3% (Table I). In patients with intracranial complications a higher incidence (20.8%) was observed. The anaerobic isolates consisted of a similar variety of micro-organisms to that reported in other studies.7-9,12 The most frequently isolated anaerobes were Peptostreptococcus (6.4%) and Prevotella species (4.7%). Some authors report an absence of anaerobes that is in part attributed to lack of anaerobic culture tests.2,4,11
Fifty-six out of 163 (34.4%) positive culture specimens were polymicrobial (Table II). Similar findings are reported in other studies.3 The implications for therapy are that bacteria with different antimicrobial resistance are frequently found within the same patient. This is illustrated in Table II by the co-existence of S. milleri, which is highly susceptible to penicillin, with penicillin-resistant micro-organisms in 18 out of 43 isolates. Polymicrobial cultures, along with the high prevalence of S. aureus and coagulase-negative staphylococci, strongly suggest that acute exacerbation of chronic sinusitis is the cause of the disease in the affected patients.9
In addition to other essential treatments, patients presenting with complications must receive intravenous antimicrobials that cover the most likely pathogens. As shown in this and other studies, the causative pathogens (Table II) and, in all likelihood, resistance patterns vary from one hospital to another.1,4-6 Recommendations for initial therapy should therefore be based on an up-to-date hospital database of pathogens and resistance patterns. Two therapeutic approaches are described; a conservative 'traditional' approach and a more radical 'de-escalation' approach. Both regimens are modified on the basis of subsequent microbiology reports. The traditional approach advocates the use of a narrow-spectrum antibiotic, with broad-spectrum drugs held in reserve for patients who are severely ill, do not respond to treatment or are immunocompromised.4 The de-escalation approach promotes the use of broad-spectrum antibiotics.1,3,5,11 In one published report the authors recommended the empiric use of a combination of vancomycin, a third- or fourth-generation cephalosporin, and metronidazole or clindamycin.3
At JH and CHB a de-escalation approach is used, with drugs that are effective against both β-lactamase-positive aerobic bacteria and anaerobes.1,4,8,11 For aerobic bacteria this typically includes either amoxicillin plus clavulanate or ampicillin plus cloxacillin. Erythromycin is used in patients with penicillin sensitivity and cephalosporins are held in reserve. Anaerobic cover is provided by metronidazole. In patients with intracranial complications a combination of penicillin, chloramphenicol and metronidazole is often used. The low prevalence of MRSA (1.3%) in this study does not warrant the empiric use of vancomycin.
Conclusion
Selection of drugs for empiric antibiotic therapy in patients with acute complicated sinusitis should be supported by knowledge of the prevalence and antimicrobial susceptibilities of bacteria isolated from patients.
S. milleri, S. aureus, β-haemolytic streptococci, coagulasenegative staphylococci, H. influenzae and the anaerobes Peptostreptococcus and Prevotella species are common causative pathogens of acute complicated bacterial sinusitis at the two hospitals studied. H. influenzae was a more frequent pathogen in children. S. pneumoniae and M. catarrhalis did not appear to be major pathogens. A significant difference in the prevalence of major pathogenic bacteria between the two hospitals is demonstrated and highlights the need for medical practitioners to be aware of the trends in their own locality.
The high incidence of polymicrobial disease (34.4%) coupled with the severity of complicated sinusitis present an argument in favour of a de-escalation approach to antimicrobial therapy. A choice of metronidazole in combination with either amoxicillin-clavulanate or ampicillin plus cloxacillin provides sufficient antimicrobial cover against the locally prevalent micro-organisms at JH and CHB.
Similar studies should be performed on a regular basis at hospitals involved in the management of acute complicated sinusitis. Continuous monitoring of the prevalence and detection of the emergence of antimicrobial-resistant bacteria will guide hospital treatment policy.
I acknowledge Professor Pradip Modi for his advice on the study design and data analysis, Dr Razwi Ahmed for facilitating data collection, and Professor James Loock for his constructive editorial comments during the final preparation of the manuscript.
References
1. Snyman J, Classen AJ, Botha PL. A microbiological study of acute maxillary sinusitis in Bloemfontein. S Afr Med J 1988; 74: 444-445. [ Links ]
2. Tellez I, Alba LMD, Reyes MG, Patton E, Hesles HdlG. Microbiology of acute sinusitis in Mexican patients. Arch Med Res 2006; 37: 395-398. [ Links ]
3. Glickstein JS, Chandra RK, Thompson JW. Intracranial complications of pediatric sinusitis. Otolaryngol Head Neck Surg 2006; 134 (5): 733-736. [ Links ]
4. Mortimore S, Wormald PJ, Oliver S. Antibiotic choice in acute and complicated sinusitis. J Laryngol Otol 1998; 112: 264-268. [ Links ]
5. Oxford LE, McClay J. Complications of acute sinusitis in children. Otolaryngol Head Neck Surg 2005; 133: 32-37. [ Links ]
6. Tshifularo M, Monama GM. Complications of inflammatory sinusitis in children: institutional review. South African Family Practice 2006; 48: 16-19. [ Links ]
7. Brook I. Microbiology and antimicrobial treatment of orbital and intracranial complications of sinusitis in children and their management. Int J Pediatr Otorhinolaryngol 2009; 73: 1183-1186. [ Links ]
8. Kombogiorgas D, Seth R, Athwal R, Modha J, Singh J. Suppurative intracranial complications of sinusitis in adolescence. Single institute experience and review of literature. Br J Neurosurg 2007; 21: 603-609. [ Links ]
9. Brook I, Foote PA, Frazier EH. Microbiology of acute exacerbation of chronic sinusitis. Ann Otol Rhinol Laryngol 2005; 114: 573-576. [ Links ]
10. Brook I. Bacteriology of acute and chronic ethmoid sinusitis. J Clin Microbiol 2005; 43(7): 3479-3480. [ Links ]
11. Ali A, Kurien M, Mathews SS, Mathew J. Complications of infective rhinosinusitis: experience from a developing country. Singapore Med J 2005; 46: 540-544. [ Links ]
12. Brook I. Microbiology of intracranial abcesses and their associated sinusitis. Arch Otolaryngol Head Neck Surg 2005; 131: 1017-1019. [ Links ]
13. Rosenfeld EA, Rowley AH. Infectious intracranial complications of sinusitis, other than meningitis, in children: 12 year review. Clin Infect Dis 1994; 18: 750-754. [ Links ]
14. Mortimore S, Wormald PJ. The Groote Schuur hospital classification of the orbital complications of sinusitis. J Laryngol Otol 1997; 111: 719-723. [ Links ]
15. Chin TO, Syrimi M, Ne SC, Twomey J. Group C Streptococcal subperiosteal abscess of the orbit. Orbit 2009; 28: 160-161. [ Links ]
Accepted 3 March 2010.
Corresponding author: I P Olwoch (olwoch@hotmail.com)














