Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.100 no.3 Pretoria Mar. 2010
ORIGINAL ARTICLES
Hutchinson's sign as a marker of ocular involvement in HIV-positive patients with herpes zoster ophthalmicus
M van DykI; D MeyerII
IMB ChB, MMed, FCOphth (SA). Division of Ophthalmology, Faculty of Health Sciences, Stellenbosch University, Tygerberg, W Cape
IIMB ChB, FCFP (SA), BSc (Hons), MMed, FCOphth (SA), PhD. Division of Ophthalmology, Faculty of Health Sciences, Stellenbosch University, Tygerberg, W Cape
ABSTRACT
BACKGROUND: A positive Hutchinson's sign indicates an increased risk of ocular involvement in herpes zoster ophthalmicus (HZO). We examined the sensitivity of Hutchinson's sign as an indicator of ocular involvement in a consecutive series of patients presenting with HZO.
METHODS: We conducted a descriptive observational prospective study of patients >18 years old presenting with HZO and consenting to pre- and post-test counselling and HIV and CD4 testing. A full ophthalmological examination focused on the extent of ocular involvement, and the presence of Hutchinson's sign was confirmed by two clinicians.
RESULTS: Thirty-three patients were enrolled; 29 were HIV positive, of whom 18 (62%) had not been diagnosed with HIV prior to enrolment. Of the 29 HIV-positive patients, 21 (72%) were Hutchinson's sign positive (HSP), all of whom had intra-ocular involvement (95% confidence interval 88 - 100%). Of the 8 HIV-positive, Hutchinson's sign-negative (HSN) patients, 4 did and 4 did not display intra-ocular involvement. Neither the mean CD4 count nor the average age in the HSP group differed significantly from the HSN group.
CONCLUSION: We confirmed that a Hutchinson's sign- and HIVpositive patient with HZO has a very high positive predictive value for intra-ocular involvement. Neither age nor CD4 count had predictive value for ocular involvement. Young adults presenting with HZO should be suspected of having HIV, and HIV-positive patients with HZO but HSN may still have ocular involvement. All patients with HZO should be seen by an ophthalmologist.
Varicella zoster virus (VZV) causes two distinct viral syndromes. VZV infection is a common and usually benign childhood infection, manifesting as chickenpox. Herpes zoster ophthalmicus (HZO) is a potentially devastating visual disease with variable presentation caused by the re-activation of a latent infection of the trigeminal ganglion by the VZV.
The most common causes of re-activation of VZV are decreased cell-mediated immunity related to age, malignancies, chemo- or radiotherapy, HIV infection, and the use of immunosuppressive drugs.1 The characteristic vesicular cutaneous involvement in the area supplied by the ophthalmic division of the trigeminal nerve with respect to the horizontal midline is usually the first clinical sign and is often preceded by pain in this area caused by viral replication in the ganglion.
Hutchinson's sign results from the involvement of the skin and/or nasal mucosa of the tip of the nose on the ipsilateral side of the HZO infection. This involvement of the external nasal nerve (the terminal branch of the nasociliary branch of the ophthalmic division of the trigeminal nerve) implies a high risk of involvement of the earlier ciliary branches supplying intra-ocular structures. This relationship between involvement of the external nasal nerve and increased likeliness of intraocular involvement was first described by John Hutchinson in 1865.2
HZO is much more common in immunocompromised patients3,4 and especially the HIV-infected population. The risk of HZO is up to 15 times higher in HIV-positive than HIV-negative patients.5,6 One study6 found the incidence of HZO in the HIV-positive population to be around 3.2 per 100 patient-years of follow-up, which was almost 10 times more than the 3.4 per 1 000 patient-years of follow-up in the general population. The incidence of HZO in HIV cases has not changed significantly since the advent of highly active antiretroviral therapy (HAART).7
The most common vision-affecting complications of HZO are corneal opacities due to scarring, neovascularisation, neurotrophic or secondarily infected ulcers, the sequelae of uveitis (i.e. cataract and glaucoma), necrotising retinitis, posterior vascular occlusions and optic neuritis.8 The risk of ocular complications of HZO is 2 - 3 times higher in the HIV-infected population than in the general population; however, the complication rate has declined slightly since the advent of HAART.9
HZO has been described as one of the presenting signs of HIV disease.10 It is a powerful predictor of HIV positivity, especially in black Africans, with figures of between 50% and 95%.4,10,11 No studies were found in the English literature on the predictive value of Hutchinson's sign for intra-ocular involvement in the HIV-positive population. However, when both the infratrochlear and the external nasal nerve (not Hutchinson's sign) were involved, 100% of patients developed intra-ocular involvement.12
The effect of CD4 count on HZO is not fully understood. Some studies demonstrated no increased incidence of HZO with duration of HIV and presumably declining CD4 count.7,13-16 It has been suggested that HZO occurs in patients with moderate degrees of immunodeficiency, and much less so in patients with profound immunodeficiency,7,13,16,17 which would suggest that, in contrast to opportunistic infections such as cytomegalovirus that occur in profound immunodeficiency, HZO rather occurs in immunodysregulation.18
We aimed to evaluate the sensitivity of Hutchinson's sign as a marker of ocular involvement in a series of consecutive HZO cases in a population at risk for HIV disease, and to explore the relationship between CD4 counts in the HIV-positive subgroup with and without a positive Hutchinson's sign.
Methods
We conducted a descriptive observational study of patients with HZO referred to the Division of Ophthalmology, Tygerberg Academic Hospital, Tygerberg, Western Cape, during 2007. The study was approved by the Ethics Committee of the Bioethics Unit, Stellenbosch University, Western Cape. Patients were included if they were >18 years old, able to give informed consent, and willing to undergo HIV and CD4 serological testing, participate in the study and return for a follow-up visit. Age <18 and refusal to consent to HIV/CD4 serological testing or refusal to participate, were exclusion factors.
Patients underwent a routine ophthalmic examination, specifically looking for the sequelae of HZO and its eye involvement. A second ophthalmologist confirmed the presence or absence of Hutchinson's sign, to eliminate misdiagnosis.
After obtaining written consent and pre-counselling, HIV serology and CD4 counts were dispatched to the local National Health Services laboratory with standard protocols for confirmation.
All patients underwent HIV post-test counselling, and were referred for antiretroviral therapy where appropriate. Age, time since initial diagnosis of HIV, presence of HAART, and extent of ocular involvement were documented. Conjunctivitis alone was not regarded as representing intra-ocular involvement. All patients received standard oral antiherpetic treatment in accordance with departmental protocols and the extent of the disease.
Statistical analysis
Data were evaluated and statistically processed; a 95% confidence interval (CI) was calculated, using binomial distribution.
Results
Of the 33 patients enrolled, 29 were HIV positive, of whom 18 (62%) were first diagnosed as HIV positive at the time of the study. Of the 11 patients previously known to be HIV positive, only 2 were on HAART on presentation (Table I).
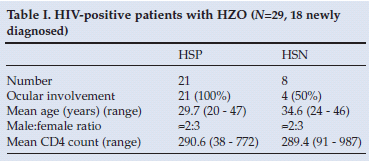
Of the 29 HIV positive patients, 21 (72%) were Hutchinson's sign positive (HSP). All 21 (100%) of these patients had intraocular involvement (95% CI 88 - 100%). Of the 8 HIV-positive but Hutchinson's sign-negative (HSN) patients, 4 had intraocular involvement and 4 did not (50% for each sub-group).
Average CD4 count in the HSP sub-group was 290.6 (range 38 - 772). This did not differ significantly from the HSN sub-group (289.4; range 91 - 897). The mean age of the HIV-positive, HSP patients was 29.7 years (range 20 - 47), and 34.6 years (range 24 - 46) for the HIV-positive, HSN cases (Table I). This difference was not statistically significant. Of the 21 HIV-positive and HSP patients, 13 (62%) were female. Of the total group of 33 patients, 24 (73%) were black, 7 (21%) were coloured and 2 (5%) were white.
Discussion
The magnitude of the HIV pandemic makes it one of the most important health issues in sub-Saharan Africa. Although the HIV incidence in the Western Cape is one of the lowest in the country, it is still rising - 8.5% in 2001 compared with 15.4% in 2006.19 According to the South African National HIV Survey of 2005, 13.3% of females and 8.2% of males in South Africa over the age of 2 years were HIV-positive.19 It is imperative that patients with potentially vision-threatening ocular involvement are identified as early as possible and appropriately referred for specialised eye care. Delays in treatment and inadequate medical therapy for herpes zoster are associated with more severe ocular complications and visual loss in HZO disease.20 Owing to the 6 - 15-fold increase in the incidence of HZO in HIV-positive patients compared with the general population, it can be presumed that HIV is the most common cause of VZV re-activation in the populations at risk.
Our findings confirm that a positive Hutchinson's sign in an HIV-positive patient with HZO is a specific predictor of intra-ocular involvement. Intra-ocular involvement ranges from mild keratitis or anterior uveitis to blinding disease with corneal perforation. As timely intervention may decrease the complications of this disease, all such patients should be referred to an ophthalmologist for evaluation. This step should be one of the criteria for ophthalmic referral of patients by primary level health workers, who should be made more aware of the relevance of Hutchinson's sign.
We did not find the CD4 count to be a significant predictor of ocular involvement, supporting previous findings and suggesting that HZO occurs in the setting of immunodysregulation rather than immunocompromise.7,13-16 Further studies into immunological factors that can predict ocular involvement in HZO are awaited.
Our study confirms previous observations that HZO in young patients may be the presenting sign of HIV positivity, as 62% of our patients with HZO were diagnosed for the first time as HIV positive at the time of HZO presentation. It confirms previous findings that HZO in a young, especially black African, patient is very suggestive of underlying retroviral disease.10
There was no difference in average age between the group with ocular involvement and the group without, and no identifiable link between age and severity of disease. The higher incidence among females probably reflects the increased incidence of HIV in females in the broader population and is in keeping with the South African National HIV Survey 200519 demographics.
As with all patients, HIV-positive patients with HZO and a negative Hutchinson's sign may still develop intra-ocular involvement. Hutchinson's sign therefore has a poor negative predictive value. All patients should be thoroughly examined for any signs of intra-ocular involvement.
References
1. Johnson RW, Dwarkin RH. Treatment of herpes zoster and post-herpetic neuralgia. BMJ 2003; 326: 748-750. [ Links ]
2. Tomkinson A, Roblin DG, Brown MJ. Hutchinson's sign and its importance in rhinology. Rhinology 1995; 33(3): 180-182. [ Links ]
3. Margolis TP, Milner MS, Shama A, Hodge W, Seiff S. Herpes zoster ophthalmicus in patients with immunodeficiency virus infection. Am J Ophthalmol 1998; 125: 285. [ Links ]
4. Umeh RE. Herpes zoster ophthalmicus and HIV infection in Nigeria. Int J Stan AIDS 1998; 9(8): 476-479. [ Links ]
5. Wiafe B. Herpes zoster ophthalmicus in HIV/AIDS. Community Eye Health 2003; 16(47): 35-36. [ Links ]
6. Hodge WG, Seiff SR, Margolis TP. Ocular opportunistic infection incidences among patients who are HIV positive compared to patients who are HIV negative. Ophthalmology 1998; 105(5): 895-900. [ Links ]
7. Veenstra J, Krol A, Van Pragg RM, et al. Herpes zoster immunological deterioration and disease progression in HIV-1 infection. AIDS 1995; 9: 1153-1158. [ Links ]
8. Rogues AM, Dupon M, Ladner J, et al. Herpes zoster and human immunodeficiency virus infection: a cohort of 101 coinfected patients. Groupe d'Epidemiologie clinique du SIDA en Aquitaine. J Infect Dis 1993; 168: 245. [ Links ]
9. Adepoji FG, Olawumi HO, Adehoya BJ. HIV seropositivity and related eye disease in Utih, Ilorin. Nigr Postgrad Med J 2007; 14(2): 163-165. [ Links ]
10. Palexas GN, Welsh NH. Herpes zoster ophthalmicus: an early pointer to HIV positivity in young African patients. Scand J Immunol Suppl 1992; 11: 67-68. [ Links ]
11. Vafai A, Berger M. Zoster in patients infected with HIV: a review. Am J Med Sci 2001; 321: 372-380. [ Links ]
12. Zaal MJ, Völker-Dieben HJ, D'Amaro J. Prognostic value of Hutchinson's sign in acute herpes zoster ophthalmicus. Graefes Arch Clin Exp Ophthalmol 2003; 241(3): 187-191. [ Links ]
13. Glesby MJ, Moore RD, Chaisson RE. Herpes zoster in patients with advanced human immunodeficiency virus infection treated with zidovudine. Zidovudine Epidemiology Study Group. J Infect Dis 1993; 168: 1264-1268. [ Links ]
14. Farizo KM, Buehler JW, Chamberland ME, et al. Spectrum of disease in persons with human immunodeficiency virus infection in the United States. JAMA 1992; 267: 1798-1805. [ Links ]
15. Alliegro MB, Dorrucci M, Pezzotti P, et al. Herpes zoster and progression to AIDS in a cohort of individuals who seroconverted to human immunodeficiency virus. Clin Infect Dis 1996; 23: 990-995. [ Links ]
16. Glesby MJ, Moore RD, Chaisson RE. Clinical spectrum of herpes zoster in adults infected with human immunodeficiency virus. Clin Infect Dis 1995; 21: 370-375. [ Links ]
17. Perronne C, Lazanas M, Leport C, et al. Varicella in patients infected with the human immunodeficiency virus. Arch Dermatol 1990; 126: 1033-1036. [ Links ]
18. Veenstra J, Krol A, Van Pragg RM, et al. Herpes zoster immunological deterioration and disease progression in HIV-1 infection. AIDS 1995; 9: 1153-1158. [ Links ]
19. Shaikh W, Abdullah F, Lombard CJ, Smit L, Bradshaw D, Makubale L. Masking through averages - interprovincial heterogeneity in HIV prevalence in Western Cape. S Afr Med J 2006; 96(6): 538-543. [ Links ]
20. Gebo KA, Kolyani R, Moore RD, Polydefkis MJ. The incidence of, risk factors for, and sequelae of Herpes Zoster among HIV patients in HAART era. J Acquir Immune Defic Syndr 2005; 40(2): 169-174. [ Links ]
21. Liesegang TJ. Herpes zoster ophthalmicus: Natural history, risk factors, clinical presentation and morbidity. Ophthalmology 2008; 115 (suppl): 3-12. [ Links ]
Accepted 18 August 2009
Corresponding author: M van Dyk (mariusvandyk@hotmail.com)














