Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.100 no.3 Pretoria Mar. 2010
ORIGINAL ARTICLES
Non-alcoholic fatty liver disease (NAFLD) in the Western Cape: A descriptive analysis
F C KrugerI; C DanielsII; M KiddIII; G SwartIV; K BrundynV; C van RensburgVI; M J KotzeVII
IMB ChB, MMed (Int), PhD (Int), Cert Gastroenterology (SA). Gastroenterology and Hepatology Unit, Department of Internal Medicine, Stellen bosch University, Tygerberg, and Durbanville Medi-Clinic, Durbanville, W Cape
IIBA (Cur). Gastroenterology and Hepatology Unit, Department of Internal Medicine, Stellen bosch University, Tygerberg, and Durbanville Medi-Clinic, Durbanville, W Cape
IIIPhD. Centre for Statistical Consultation, Stellenbosch University
IVMB ChB, MMed (Anat Path) K Brundyn, MB ChB, MMed (Anat Path). Department of Pathology, Stellenbosch University, and National Health Laboratory Services, Anatomical Pathology, Tygerberg Hospital, Tygerberg, W Cape
VMB ChB, MMed (Anat Path). Department of Pathology, Stellenbosch University, and National Health Laboratory Services, Anatomical Pathology, Tygerberg Hospital, Tygerberg, W Cape
VIMB ChB, MMed (Int), PhD (Int). Gastro-enterology and Hepatology Unit, Department of Internal Medicine, Stellenbosch University
VIIPhD. Department of Pathology, Stellenbosch University
ABSTRACT
BACKGROUND: Non-alcoholic fatty liver disease (NAFLD) is the most prevalent chronic liver disease in Western countries, but the disease profile has not yet been described in South Africa. NAFLD affects all spheres of society, especially the poorest and least educated.T
AIM: To investigate the demographics and clinical and biochemical features of South African patients diagnosed with non-alcoholic fatty liver and non-alcoholic steatohepatitis (NASH) in the Western Cape, South Africa.
DESIGN/METHOD: Overweight/obese subjects were screened by ultrasound and those with fatty liver/hepatomegaly were included. Liver biochemistry, insulin resistance (using the insulin resistance homeostasis model assessment method for insulin resistance, HOMA-IR) and body mass index were assessed and liver biopsies were performed on patients older than 45 years with persistently abnormal liver function and/ or hepatomegaly.
RESULTS: We screened 233 patients: 69% coloured, 25% Caucasian, 5% black and 1% Asian. The majority (73%) were female. NAFLD was confirmed histologically in 111 patients, of whom 36% had NASH and 17% advanced liver fibrosis. No black patient had advanced fibrosis. Subjects with NASH had higher mean triglyceride (p=0.03) and cholesterol (p=0.01) levels than subjects with NAFL. All patients were insulin resistant/diabetic. HOMA-IR and not the degree of obesity was strongly associated with advanced fibrosis (p=0.09).
CONCLUSION: This study is the first to describe the clinical characteristics of NAFLD in South Africa, albeit only in the Western Cape population. Insulin resistance was the universal factor present. The degree of obesity was not associated with severity of disease. The role of genetic risk factors in disease development and severity remains to be defined.
Non-alcoholic fatty liver disease (NAFLD) is a complex disorder characterised by histological abnormalities identified on liver biopsy in patients who consume little or no alcohol but present with histological features similar to those seen in alcoholic liver disease. NAFLD is the most prevalent chronic liver disease in Western countries. In the USA the estimated prevalence is 17 - 33%.1
Non-alcoholic steatohepatitis (NASH) is a subset of NAFLD. The estimated prevalence of NASH in the USA is 6 - 17%.1 NASH is not a benign liver disease - it may result in increased rates of cirrhosis, hepatocellular carcinoma, liver transplantation and death.2 NASH increases in parallel with increases in obesity and sedentary lifestyle.3 The risk of developing cirrhosis from NASH is approximately 20% over 10 years. The National Institute of Health (NIH) in the USA found the highest rates of obesity among the least educated with the highest poverty rates.4 This finding may be of relevance to the South African population, as the overall prevalence of overweight (body mass index (BMI) >25) and obesity (BMI >30) in South Africa is very high, with more than 29% of men and 56% of women being classified as overweight or obese.5
The major risk factors for advanced NAFLD include age (>45 - 50 years), overweight/obesity, insulin resistance (even in young, non-obese individuals) and ethnicity.6 The prevalence of NAFLD/NASH in African Americans appears to be lower than that for other population groups in the USA, despite their over-representation of the major risk factors.7,8 Ethnicity as a major risk factor for NASH has also been highlighted by a postmortem paediatric study in the USA showing that there are distinct histopathological differences in NAFLD/NASH between Caucasians and the minority race groups.9 African Americans were also poorly represented in this study, again highlighting their low prevalence of NAFLD/NASH.
Ethnic differences suggest a role for genetic factors in susceptibility to developing advanced NAFLD, which is supported by family studies. Numerous candidate genes involved in the development and progression of this condition have been investigated.10,11
We aimed to describe the clinical characteristics, demographics and biochemical features in South African patients in the Western Cape screened for and diagnosed with NAFLD/NASH.
Subjects and methods
The study protocol was approved by the Ethics Review Committee of Stellenbosch University, and all patients included signed informed consent.
We included patients who attended the clinics at the Gastroenterology and Hepatology Unit of the Division of Internal Medicine at Tygerberg Hospital and Louis Leipoldt and Durbanville Medi-Clinics. Between July 2003 and December 2006, 233 overweight/obese patients with features suggestive of the metabolic syndrome were screened for hepatic steatosis by ultrasonography.
Study design
The following aspects were investigated: gender, age, ethnicity, total cholesterol and triglyceride levels, type 2 diabetes mellitus (DM2), insulin resistance (using the insulin resistance homeostasis model assessment method for insulin resistance, HOMA-IR) and BMI. Known diabetics were excluded from HOMA-IR calculations.
Subjects were grouped according to those who fulfilled the criteria for NASH and NAFL. NAFL is defined as steatosis only or steatosis with inflammation but not fulfilling the criteria for NASH. The subjects with histological features suggestive of another liver disease were excluded. Subjects were also grouped according to the severity of fibrosis: advanced fibrosis (grade 3 and 4) or no/mild fibrosis (grade 0, 1 and 2), according to Kleiner et al.12
Biochemical parameters
Liver function, serum glucose, serum insulin and lipograms were determined after an overnight fast, using standard laboratory techniques. Additional blood tests were performed where necessary to exclude other liver diseases. HOMA-IR was calculated as follows: fasting glucose × fasting insulin/22.5.
Anthropometric parameters
Height and weight measurements were recorded from which BMIs were calculated as follows: mass in kg/ length in m2.
Liver biopsies
Liver biopsies were performed percutaneously under ultrasound guidance, or laparoscopically during cholecystectomy for patients with ultrasonographic evidence of steatosis, on patients older than 45 years with fatty infiltration on ultrasound and/or who had persistently abnormal liver function and/or hepatomegaly.
Histological analysis
Liver biopsies were reviewed by the same two pathologists using the criteria of the NIH Non Alcoholic Steatohepatitis Clinical Research Network (NIH NASH CRN).12
Statistical analysis
For descriptive purposes, cross-tabulation and frequency tables were used to describe occurrences of various attributes (e.g. gender, ethnicity, etc.) of the sample. Comparisons between different groupings of the sample for measurements (e.g. BMI, HOMA-IR, etc.) were done using one-way analysis of variance (ANOVA). A 5% significance level was used as guideline for determining significant differences.
Results
The subjects screened were ethnically classified as coloured (69%), Caucasian (25%), black (5%) and Asian (1%). The majority (73%) of the patients were female.
A total of 182 patients (87%) had ultrasonographic evidence of fatty infiltration and/or hepatomegaly. Of the group with an ultrasound scan suggesting fatty liver and who fulfilled the criteria for liver biopsy, 127 consented to biopsy. NAFLD was confirmed in 111 (87%), and of those with NAFLD 46 (36%) had NASH. Fig. 1a displays histological findings of the group that underwent liver biopsy. Sixty-three (54%) of the patients had no histological evidence of fibrosis, while 20 (17%) had advanced liver fibrosis (stages 3 - 4) (Fig. 1b). Fig. 2 illustrates the difference between the males and females affected by NAFLD with reference to NASH versus NAFL and advanced fibrosis versus no/mild fibrosis.
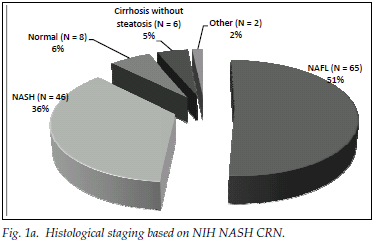
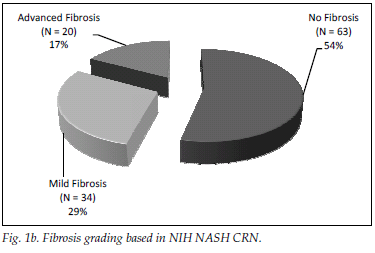
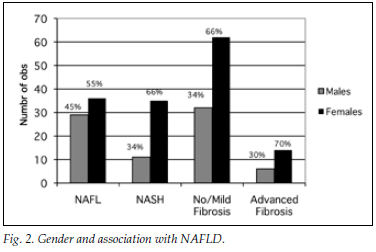
Fig. 3 illustrates the association between patient age and presentation with NAFL/NASH as well as no/mild fibrosis and advanced fibrosis. The mean age for the group with fatty liver disease was 50 years (range 48 - 53 years) versus 54 years (51 - 58 years) for NASH (p=0.1). The mean ages of the groups with fatty liver disease and NASH were 50 years (95% confidence interval (CI) 48 - 53 years) and 54 years (95% CI 51 - 58 years) (p=0.1), The mean for the group with no or mild fibrosis was 51 years (95% CI 49 - 53 years) and that for the group with advanced fibrosis 57 years (95% CI 49 - 65 years) (p=0.06).
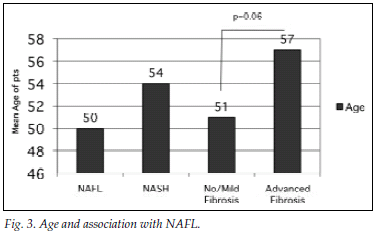
Fig. 4 illustrates the differences between the ethnic groups with regard to presentation with NASH versus NAFL and grading of fibrosis. The Asian population group was excluded from the figure, since there was only 1 patient in this group.
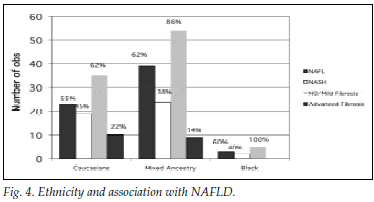
Fig. 5 illustrates the prevalence of DM2 in the different groups.
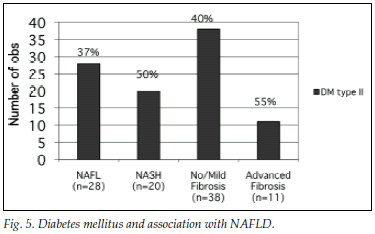
Table I displays the mean total cholesterol level (mg/dl), serum triglyceride level (mg/dl), BMI (kg/m2) and HOMA-IR for each group.
Discussion
We describe the disease profile of NAFLD/NASH for the first time in South African patients in the Western Cape region. Their clinical characteristics are described in terms of histological features and presence of fibrosis, in relation to gender, age, ethnicity, DM2, HOMA-IR and BMI. Our findings are similar to observations elsewhere in the world.13,14 However, the majority (73%) of our patients were female. Gender was not associated with severity of NAFLD in this cohort of patients.
Patients were required be 45 years and older to receive a liver biopsy. Although older age was not associated with NASH, it was associated with more advanced fibrosis. Ethnicity independently was shown to be an important risk factor for advanced disease in the USA.7,8 African American subjects appear to be protected against advanced disease.8 Our findings suggest the same, despite black patients being under-represented. This warrants further studies to draw comparisons with US studies.
Insulin resistance is the common pathogenic factor in the majority of patients with NAFLD when other causes have been excluded.13 The subjects not diagnosed with DM2 had a markedly increased mean HOMA-IR. This confirms the pathogenic role of insulin resistance in subjects with NAFLD. Even though insulin resistance was not predictive of NASH there was a strong tendency towards predicting advanced fibrosis. DM2 is an independent risk factor for more advanced disease, but our study was unable to demonstrate this.14
There was a statistically significantly difference in the mean serum triglycerides and total cholesterol levels in subjects with NAFL and NASH, but not for subjects with no/mild fibrosis compared with advanced fibrosis. These findings implicate dyslipidaemia but not advanced fibrosis in the development of NASH.
Even though all our patients were either overweight or obese, the presence of more marked obesity did not predict the presence of more advanced disease. Our findings suggest that obesity, insulin resistance and dyslipidaemia are important factors in the development of NAFL and NASH, while secondary factors involving gene-environment interactions may be required to develop advanced fibrosis.15
In conclusion, we present the first clinical description of NAFLD in a South African setting, with particular emphasis on the unique coloured population of the Western Cape. Their phenotype was more similar to that of the Caucasians than the black population group. Insulin resistance was the key factor, present in all the patients. Further studies are warranted to identify the role of genetic risk factors in the development of NAFLD and progression to advanced disease.
References
1. McCullough AJ. The epidemiology of risk factors of NASH. In: Farrel GC, George J, Hall PdelaM, McCulloughAJ, eds. Fatty Liver Disease. NASH and Related Disorders. Massachusettes: Blackwell Publishing, 2005: 23-37. [ Links ]
2. Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology 1999; 116: 1413-1419. [ Links ]
3. Garcia-Monzon C, Martin-Perez E,Iacona OL, et al. Characterization of pathogenic and prognostic factors of nonalcoholic steatohepatitis associated with obesity. J Hepatol 2000, 33: 716-724. [ Links ]
4. Drewnowski A, Specter SE. Poverty and obesity: the role of energy density and energy costs. Am J Clin Nutr 2004; 79(1): 6-16. [ Links ]
5. Puoane T, Steyn K, Bradshaw D, Laubscher R, Fourie J, Lambert V, Mbananga N. Obesity in South Africa: The South African demographic and health survey. Obesity Research 2002; 10: 1038-1048. [ Links ]
6. Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology 1990; 12: 1106-10. [ Links ]
7. Weston SR, Leyden W, Murphy R, Bass NM, Bell BP, Manos MM, Terrault NA. Racial and ethnic distribution of nonalcoholic fatty liver disease in persons with newly diagnosed chronic liver disease. Hepatology 2005; 41(2): 372-379. [ Links ]
8. Caldwell SH, Harris DM, Patrie JT, Hespenheide EE. Is NASH underdiagnosed among African Americans? Am J Gastroenterol 2002; 97: 1496-1500. [ Links ]
9. Schwimmer JB, Behling C, Newbury R, et al. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology 2005; 42: 641-649. [ Links ]
10. Willner IR, Waters B, Patil SR, Reuben A, Morreli J, Riely CA. Ninety patients with nonalcoholic steatohepatitis: insulin resistance, familial tendency, and severity of disease. Am J Gastroenterol 2001; 96: 2957-2961. [ Links ]
11. Struben VM, Hespenheide EE, Caldwell SH. Nonalcoholic steatohepatits and cryptogenic cirrhosis within kindreds. Am J Med 2000; 108: 9-13. [ Links ]
12. Kleiner DE, Brunt EM, Van Natta ML, Behling C, Contos MJ, Cummings OW. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005; 41(6): 1313-1321. [ Links ]
13. Brunt EM. Nonalcoholic steatohepatitis. Semin Liver Dis 2004; 24: 3-20. [ Links ]
14. Adams LA, Sanderson SO, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol 2005; 42: 132-138. [ Links ]
15. Day CP. Genes or environment to determine alcoholic liver disease and non-alcoholic fatty liver disease Liver Int 2006; 26(9): 1021-1028. [ Links ]
Corresponding author: F C Kruger (ckruger@gastrosa.com)














