Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
SAMJ: South African Medical Journal
versión On-line ISSN 2078-5135
versión impresa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.100 no.2 Pretoria feb. 2010
ORIGINAL ARTICLES
Cancer prevalence in 129 breast-ovarian cancer families tested for BRCA1 and BRCA2 mutations
C M SchlebuschI; G DreyerII; M D SluiterIII; T M YawitchIV; H J van den BergVI; E J van RensburgV
IBSc, BSc (Hons), MSc. Cancer Genetics, Department of Genetics, University of Pretoria
IIMB ChB, MMed (O&G), MCOG (SA). Department of Obstetrics and Gynaecology, University of Pretoria
IIIBSc, BSc (Hons), MSc. Cancer Genetics, Department of Genetics, University of Pretoria
IVBSc, BSc (Hons), MSc. Cancer Genetics, Department of Genetics, University of Pretoria
VBSc, BSc (Hons), MSc, PhD. Cancer Genetics, Department of Genetics, University of Pretoria
VIDiploma in Nursing (Community nurse). Department of Health, Gauteng Provincial Administration
ABSTRACT
BACKGROUND: Women who carry germline mutations in the breast-ovarian cancer susceptibility genes, BRCA1 and BRCA2, are at very high risk of developing breast and/or ovarian cancer. Both genes are tumour suppressor genes that protect all cells from deregulation, and there are reports of their involvement in other cancers that vary and seem to depend on the population investigated. It is therefore important to investigate the other associated cancers in different populations to assist with risk assessments.
OBJECTIVES: To assess the cancer risk profile in BRCA-mutationpositive and negative South African breast-ovarian cancer families, mainly of Caucasian origin.
DESIGN: Descriptive study in which the prevalence of all cancers in the pedigrees of BRCA1- and BRCA2-mutationpositive groups and a group of families without mutations in either gene were compared with the general population.
RESULTS: As expected, female breast and ovarian cancer was significantly increased in all three groups. Furthermore, male breast cancer was significantly elevated in the BRCA2-positive and BRCA-negative groups. Stomach cancer prevalence was significantly elevated in the BRCA2-positive families compared with the general population.
CONCLUSIONS: These results can be applied in estimation of cancer risks and may contribute to more comprehensive counselling of mutation-positive Caucasian breast and/or ovarian cancer families.
Breast cancer is the most common malignancy among women in South Africa, with a crude incidence rate of 18.5/100 000 recorded between 1993 and 1995.1 A small but significant percentage (5 - 10%) of breast cancer cases are directly due to an inherited susceptibility.2 Two tumour suppressor genes involved in early-onset breast and ovarian cancer, BRCA1 and BRCA2, have been mapped and cloned.3,4 These two genes explain 20 - 40% of heritable breast cancer cases in various populations over the world.5,6 A large linkage and mutation study on 237 families collected by the Breast Cancer Linkage Consortium found that overall BRCA1 accounts for 52% of all families, and BRCA2 for 32%, leaving 16% of the families with a familial breast cancer phenotype unaccounted for.7 In the study, 81% of the families with both a breast and an ovarian phenotype were BRCA1- positive families while 14% linked to BRCA2. The situation was reversed in families that presented with a male breast cancer phenotype in addition to female breast cancer, where 76% linked to BRCA2 and only a small percentage to BRCA1.
Worldwide many families with a strong history of familial breast cancer have been fully screened for BRCA1 and BRCA2 mutations but none were found. This is especially the case in breast cancer-specific families (no other cancers beside breast cancer in family). While the search for the BRCA1 and BRCA2 genes was helped by the strong association of ovarian cancer in addition to breast cancer with BRCA1 and male breast cancer with BRCA2, the search for other breast cancer-associated genes is more complicated. Studies on BRCA1- and 2-negative breast cancer families showed that the most probable explanation is that there are multiple additional genes with lower penetrance and/or prevalence, each responsible for a small number of families.8,9
The functions of the BRCA genes have not been fully elucidated, but they are broadly classified as tumour suppressor genes with functions in DNA repair and recombination, cell cycle regulation through checkpoint control and transcription regulation.10,11 Owing to their function in cell cycle regulation and damage response, mutations in these genes are expected to lead to susceptibility for deregulation and cancer in more than one tissue type. It is unclear why mutations in these two genes are mainly involved in malignancies in the breast and ovaries, but it is thought that some interaction with the female hormones, oestrogen and progesterone, may be responsible.12
Mutations within BRCA1 and BRCA2 have been demonstrated to contribute to an increased risk of cancers other than breast and ovarian cancer. Friedenson summarised 32 studies (involving >70 000 individuals) that investigated the elevated risks for other cancers, associated with these two genes.13 The increased risks range from 20% to 60%, with the most important increases in cancers of the stomach and pancreas. BRCA2 confers an elevated risk to a broader spectrum of cancers than BRCA1. Many reviewed studies, however, had conflicting reports, stressing the importance of contributing genetic factors and environmental influences that may differ between populations.
We studied the occurrence of different types of cancer in 127 Caucasian and 2 non-Caucasian South African families with a positive breast cancer history. Complete mutation analysis allowed division of these families into BRCA1-mutation positive, BRCA2-mutation positive and families with no mutation in either gene. The pedigrees of these three groups of families were compared to determine the respective contribution of BRCA1 and BRCA2 to breast and ovarian cancer and to establish which other cancers may be associated with mutations in these genes.
The study was approved by the Ethics Committee of the University of Pretoria (Protocol 18/98).
Methods
Selection of patients
Participating families were obtained through a familial cancer clinic at the University of Pretoria. Families willing to participate and with a family history (3 or more cases with breast and/or ovarian cancer) suggestive of inherited susceptibility were included.
We assembled a total of 129 (127 Caucasian and 2 non-Caucasian) families: 81/129 (62.8%) were Afrikaners, mainly descended from Dutch, German and French immigrants to the South African Cape during the early 17th century,14 Ashkenazi Jewish families represented 15.5 % (20/129), 12.4% (16/129) were of British/UK origin, and 2.32% (3/129) were of Dutch descent. In addition, there were single families of Afrikaner-Lebanese, Austrian, Belgian, German, German-British, Polish and Portuguese descent. The 2 non-Caucasian families were a black South African and a South African Indian family.
A blood sample from index individuals (affected with breast and/or ovarian cancer) in these families was obtained with informed consent, and the two BRCA genes were screened for mutations using single-strand conformation polymorphism and heteroduplex analysis (SSCP/HA), protein truncation test (PTT) and multiplex ligation-dependent probe amplification (MLPA) methods. Of the 129 families, 26 tested positive for a mutation in BRCA1 and 43 for a mutation in BRCA2, while 60 remained unassigned ('BRCA negative') after full screening.15,16 The three groups were analysed to determine the prevalence of cancer in family members.
Pedigree analysis
Information regarding cancer cases in the families were obtained from the index individuals and were not verified with pathological reports. The reliability of the information, especially for more distant relatives, may be lower than that for first- and second-degree relatives' cancers. Females and males included in the analysis were only from the branches of the pedigree (paternal or maternal) that were believed to carry mutations in one of the BRCA genes. Only the index cases were screened for mutations; none of the other family members in the pedigree were screened. The assumptions of the inheritance of the mutation were based primarily on the occurrence of breast and ovarian cancer in females and secondarily on other cancers in all individuals. Where there were cancers in both branches of the family, the branch with the most cancers in the closest relatives were included. For the previous generations, all males and females in the pedigrees were included, up to the generation where the first reported case appeared. Persons in more current generations were included only if they were born before 1960 (~40 years old at time of analysis). This therefore excludes very young persons who would not yet have developed cancer.
The number of females in the pedigrees was used in calculations for female-specific cancers, and the same applies for the male-specific cancers. In the case of cancer affecting both the female and male populations the unknown gender counts were included. Bilateral cases (in females and males) were counted as two separate cases, but only the age at first diagnosis was used in calculations regarding age.
For the chi-square test the expected number of cancer cases was obtained by multiplying the population risk with the total number of individuals in the group. The population risk in South African individuals for 1993 - 1995 was obtained from the National Cancer Registry (NCR).1 The Cancer Registry data are limited in that they only supply information on histopathologically confirmed tumour data and not on population-based data. These data are at best a minimal estimate of 'population' risks. Cancer risks in the Caucasian population of South Africa were used for the calculations, since the majority of the study families were of Caucasian origin (only 2 families were non-Caucasian, i.e. 1 black in the BRCAnegative group and 1 Indian in the BRCA1-positive group). For the chi-square tests for cancers, which are not gender specific, the mean between the cancer risk of Caucasian males and females in South Africa was used.
Results
Mutation-positive families constituted 53% of the total families. The BRCA2-positive families were largely site-specific breast cancer families (33/43), compared with the BRCA1-positive families (6/26) (Table I). The total number of individuals in all the pedigrees included was 3 682 with an average of 28.5 individuals per pedigree. The gender of 97% was known, with 57% being female. The total number of all cancer cases per pedigree was higher in the BRCA-positive groups than in the BRCA-negative group (Table I). The prevalence of breast cancer was high in all three groups, with the highest prevalence in the BRCA2-positive group (Table I). Bilateral breast cancer was more common in both the mutation-positive groups. Ovarian cancer occurred mostly in the BRCA1-positive group, with the BRCA2-positive and mutation-negative groups comparable (Table I). Male breast cancer and cancers other than breast and ovarian cancer were most prevalent in the BRCA2-positive group.
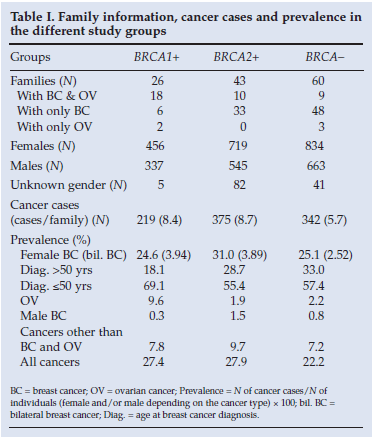
Table II compares the observed cancers in the study groups with the expected number of cancers (based on NCR data on the general Caucasian population risk1). The results concerning cancers typically associated with the two BRCA genes, namely breast and ovarian cancer for BRCA1 and breast, ovarian and male breast cancer for BRCA2, were anticipated. The observed prevalence of these cancers compared with the expected prevalence based on the general Caucasian population risk was also elevated in the mutation-negative group. This is to be expected, as the criterion on which families were selected was that they must have at least three breast and/or ovarian cancer cases. These families would therefore by default have a higher than expected prevalence of breast and ovarian cancer.
Male breast cancer had a higher prevalence than expected in the BRCA2-positive (p<0.00001) and BRCA-negative (p=0.0022) groups, but not in the BRCA1-positive group (Table II). The only other cancer with a significantly increased prevalence (p=0.0001) was stomach cancer in the BRCA2-positive group. In all three groups prostate, colon, bladder and lung cancers and melanoma had a significantly lower prevalence than expected. Overall, the prevalence of cancers in total was higher in all three study groups (p<0.00001) compared with what would be expected from the general Caucasian population data (Table II).
Discussion
Breast cancer
Breast cancer prevalences for the BRCA1-positive group and the BRCA-negative group were similar (p=0.843) (Table I). The BRCA2-positive group had a significantly higher prevalence of breast cancer than the BRCA1-positive group (p=0.015) and the BRCA-negative group (p=0.009), which seems to indicate that a higher breast cancer risk is associated with BRCA2 than with BRCA1 (Table I). Generally BRCA2 mutation penetrance for lifetime risk of breast cancer is lower than for BRCA1 mutation carriers, resulting in a later age of onset.13 This was also apparent in our study, where more BRCA1-positive individuals than BRCA2-positive individuals were diagnosed with breast cancer below the age of 50 years (Table I).
Ovarian cancer
Previously the cumulative risk for ovarian cancer in BRCA2 was established at 11% versus 39% for BRCA1.17 A study on breast ovarian cancer families with at least two cases of ovarian cancer showed these families to be four times more likely to carry a BRCA1 mutation than a BRCA2 mutation.18 Likewise, in our study 9.6% of individuals in the BRCA1-positive group had ovarian cancer compared with 1.9% in the BRCA2-positive group (Table I), indicating a significant difference between the two groups (p<0.001).
Male breast cancer
Our study supported our expectation that a BRCA2-mutationpositive genotype would result in fewer ovarian cancer cases and more male breast cancer cases than in the BRCA1-positive group, as in other studies7,19 (Table I). Interestingly, in the BRCA-negative group the male breast cancer prevalence also appeared to be increased compared with what was expected for the general population (Table II). However, this may not be a true reflection as we selected families for the presence of male and female breast cancers. In the BRCA-negative group the 5 male cases each came from different families (1 case per family), whereas the 8 breast cancer cases observed in the BRCA2-positive group came from only 3 families (2.7 cases per family). It was reported previously that once an index case presents with male breast cancer in a BRCA2-positive family a number of other cases also emerge in the rest of the family.20 This could possibly indicate gene-gene interactions that modify penetrance of male breast cancer in certain BRCA2-positive families.
Other cancers
Of the other cancers recorded in the families, only stomach cancer in the BRCA2-positive group showed a significantly increased prevalence compared with the general population (Table II).
Prostate, colon, bladder and lung cancers and melanoma appear to have a lower than expected prevalence in all three groups, probably because of incomplete reports of all cancers in the families. Some of these cancers have a high population risk, e.g. prostate cancer with a 1 in 14 lifetime risk. Incomplete reporting will affect the cancers with a higher population risk to a greater extent than those with a lower risk. To illustrate the point: if 30% of all cancers in a group of 1 346 individuals (similar in size to the BRCA2-positive group) were not reported, it would lead to 11 cases of unreported colon cancer (risk 1/35.5) while only 1 case of brain cancer (risk 1/339.5) would not be reported.
Another consideration could be that the under-reported cases are not the same over all the cancer categories but that there are preferential reports on breast and ovarian cancer, as this study focused on these cancers. The clinic that collected the families for this study tried to limit this tendency by informing patients of the importance of recording all cancers before gathering their pedigree information. However, a bias may still remain and will persist in studies that rely on secondary information supplied by the family rather than on hospital and pathology records.
Table III presents reports13 that showed associations with various types of cancers compared with our study. As many of the cancers in our study had few reported cases, one must be careful to attach significance to the difference in prevalence between the groups. However, stomach and prostate cancer each had 40 or more reported cases. Stomach cancer had a significantly (p=0.0001) elevated prevalence in the BRCA2 families compared with the general population and had twice the prevalence compared with the BRCA1-positive group. Although prostate cancer was not significantly elevated in the BRCA-mutation-positive groups compared with the general population, it is still interesting that the BRCA2-positive group had a 5 times higher prevalence compared with the BRCA1positive group (Table III).

The BRCA-negative group
Just what the families that constitute the BRCA-negative group signify is uncertain. They may represent a diverse group of families with the hypothetical BRCA3 gene, families with genes of lower penetrance, families with mutations in high-penetrance genes that are very rare, or just families with BRCA1 or 2 mutations that were missed by the methods employed. Our methods are supposed to have a 71 - 79% sensitivity,21 giving an estimate of 27 - 37 families in the BRCA - negative group, which might have BRCA1/2 mutations that were missed by the current screening methods. However this still leaves 23 - 33 families in which the breast cancer phenotype can be ascribed to either rare high-penetrant genes or low-penetrant genes that might segregate in these families together with polymorphisms in other genes that enhance cancer penetrance.
Conclusion
BRCA2 has been implicated in increased susceptibility to a larger range of cancers than BRCA1. This was also seen in our study, where the prevalence of breast, ovarian, male breast and stomach cancer was significantly increased in the BRCA2-positive group. Although cancers of the liver, prostate and uterus were more prevalent in the BRCA2-positive group than the other two groups, this was not significantly elevated compared with the general population.
Both BRCA1 and BRCA2 genes play an important role in the aetiology of familial breast cancer in South Africa. Reports have suggested mutation-specific cancer prevalence for the classically BRCA-associated cancers and other cancers.13 We report on the cancer risk profile in BRCA-mutation-positive and negative Caucasian breast-ovarian cancer families collected in South Africa. Our results regarding cancer risks in BRCA-mutation-positive families largely agree with published data. This allows for more comprehensive counselling of Caucasian mutation-positive breast and breast-ovarian cancer families regarding their risks of breast-ovarian cancer and of other associated cancers.
We thank the families for their participation in the study, Marlene de la Rey for the DNA extractions, and the Cancer Association of South Africa (CANSA) for financial support to EJvR.
References
1. Sitas F, Madhoo J, Wessie J. Incidence of Histologically Diagnosed Cancer in South Africa, 1993-1995. Johannesburg: National Cancer Registry of South Africa, 1998. [ Links ]
2. Diamond TM, Sutphen R, Tabano M, Fiorica J. Inherited susceptibility to breast and ovarian cancer. Curr Opin Obstet Gynecol 1998; 10(1): 3-8. [ Links ]
3. Miki Y, Swensen J, Shattuck-Eidens D, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 1994; 266: 66-71. [ Links ]
4. Wooster R, Bignell G, Lancaster J, et al. Identification of the breast cancer susceptibility gene BRCA2. Nature 1995; 378: 789-792. [ Links ]
5. Wooster R, Weber BL. Breast and ovarian cancer. N Engl J Med 2003; 348:2339-2347. [ Links ]
6. Thompson D, Easton D. The genetic epidemiology of breast cancer genes. J Mammary Gland Biol Neoplasia 2004; 9(3): 221-236. [ Links ]
7. Ford D, Easton DF, Stratton M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet 1998; 62(3): 676-689. [ Links ]
8. Nathanson KL, Weber BL. Other' breast cancer susceptibility genes: searching for more holy grail. Hum Mol Genet 2001; 10(7): 715-20. [ Links ]
9. Antoniou AC, Easton DF. Models of genetic susceptibility to breast cancer. Oncogene 2006; 25(43): 5898-5905. [ Links ]
10. Gudmundsdottir K, Ashworth A. The roles of BRCA1 and BRCA2 and associated proteins in the maintenance of genomic stability. Oncogene 2006; 25(43): 5864-5874. [ Links ]
11. Boulton SJ. Cellular functions of the BRCA tumour-suppressor proteins. Biochem Soc Trans 2006; 34(Pt 5): 633-645. [ Links ]
12. Hulka BS, Moorman PG. Breast cancer: hormones and other risk factors. Maturitas 2001; 38(1): 103-113; discussion 113-116. [ Links ]
13. Friedenson B. BRCA1 and BRCA2 pathways and the risk of cancers other than breast or ovarian. Medscape General Medicine 2005; 7(2): 60. [ Links ]
14. Greeff JM. Deconstructing Jaco: genetic heritage of an Afrikaner. Ann Hum Genet 2007; 71(Pt 5): 674-688. [ Links ]
15. Reeves MD, Yawitch TM, van der Merwe NC, van den Berg HJ, Dreyer G, van Rensburg EJ. BRCA1 mutations in South African breast and/or ovarian cancer families: evidence of a novel founder mutation in Afrikaner families. Int J Cancer 2004; 110(5): 677-682. [ Links ]
16. van Rensburg EJ, van der Merwe NC, Sluiter MD, Schlebusch CM. Impact of the BRCA-genes on the burden of familial breast/ovarian cancer in South Africa [Abstract 382]. Presented at the annual meeting of the American Society of Human Genetics, October 2007, San Diego, California.http://www.ashg.org/cgi-bin/ashg07s/ashg07?author=van%20Rensburg&sort=ptimes&sbutton=Detail&absno=21084&sid=737626 (accessed 31 January 2009). [ Links ]
17. Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet 2003; 72(5): 1117-1130. [ Links ]
18. Gayther SA, Russell P, Harrington P, Antoniou AC, Easton DF, Ponder BA. The contribution of germline BRCA1 and BRCA2 mutations to familial ovarian cancer: no evidence for other ovarian cancer-susceptibility genes. Am J Hum Genet 1999; 65(4): 1021-1029. [ Links ]
19. Bishop DT. BRCA1 and BRCA2 and breast cancer incidence: a review. Ann Oncol 1999; 10: Suppl 6, 113-119. [ Links ]
20. Johannsson O, Loman N, Moller T, Kristoffersson U, Borg A, Olsson H. Incidence of malignant tumours in relatives of BRCA1 and BRCA2 germline mutation carriers. Eur J Cancer 1999; 35(8): 1248-1257. [ Links ]
21. Evans DG, Bulman M, Young K, Gokhale D, Lalloo F. Sensitivity of BRCA1/2 mutation testing in 466 breast/ovarian cancer families. J Med Genet 2003; 40(9): e107. Accepted 10 June 2009. [ Links ]
Accepted 10 June 2009.
Corresponding author: C M Schlebusch (cschlebu@gmail.com)














