Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.100 no.1 Pretoria Jan. 2010
ORIGINAL ARTICLES
Treatment of trichomoniasis in pregnancy in sub-Saharan Africa does not appear to be associated with low birth weight or preterm birth
Elizabeth StringerI, *; Jennifer S ReadII; Irving HoffmanIII; Megan ValentineIV; Said AboudV; Robert L GoldenbergVI
IMD, Department of Obstetrics and Gynecology, University of Alabama at Birmingham, Birmingham, Ala., USA, and Centre for Infectious Disease Research in Zambia, Lusaka, Zambia
IIMD, MS, MPH, DTM&H, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Md., USA
IIIPA, MPH, Department of Medicine, University of North Carolina, Chapel Hill, NC, USA
IVPA-C, MS, Family Health International, Durham, NC, USA
VMD, MMed, Department of Microbiology and Immunology, Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania
VIMD, Department of Obstetrics and Gynecology, Drexel University College of Medicine, Philadelphia, Penn., USA
ABSTRACT
OBJECTIVES: To determine whether treatment of trichomoniasis increases the risk of prematurity.
DESIGN: Sub-analysis of a randomised trial.
SETTING: We analysed data from HPTN 024, a randomised trial of antenatal and intrapartum antibiotics to reduce chorioamnionitis-related perinatal HIV transmission.
SUBJECTS: Pregnant women from four sites in Africa.
OUTCOME MEASURES: Gestational age at the time of delivery or mean birth weight.
RESULTS: Of 2 428 women-infant pairs included, 428 (18%) had trichomoniasis at enrolment. There were no differences in infant age or birth weight between women with or without trichomoniasis. By randomisation group, there were no differences in gestational age at birth or birth weight. Of the 428 women diagnosed with trichomoniasis, 365 (83%) received antibiotics and 63 (15%) did not. In analysis of actual use of antibiotics, women with trichomoniasis who received no treatment were more likely to deliver a preterm infant when the symphysis-fundal height was used to estimate gestational age (36% v. 23%; p=0.03), but not when the Ballard score was used (16% v. 21%; p=0.41). There were no differences in mean birth weight between groups.
CONCLUSIONS: In pregnant women in sub-Saharan Africa, most of whom were HIV-infected, neither trichomoniasis nor its treatment appears to influence the risk of preterm birth or a low-birth-weight infant.
Preterm birth is an important cause of neonatal morbidity and mortality worldwide.1,2 Studies have suggested that bacterial infections of the placenta and fetal membranes may play a major role in preterm birth.3,4 Although the most common micro-organisms associated with preterm birth are Ureaplasma urealyticum, Mycoplasma hominis, Gardnerella vaginalis, Bacteroides species, Mobiluncus curtsii and a variety of anaerobic species, some studies have suggested that infection with Trichomonas vaginalis may increase the risk of preterm birth.5 A high prevalence of trichomoniasis among adults in eastern and southern Africa, ranging from 17% to 42%, has been reported.6-8 Use of antibiotics to prevent preterm labour and premature membrane rupture, or to reduce the risk of preterm birth for women in preterm labour, has been widely studied for over 30 years. About a third of the studies have suggested benefit in reducing preterm birth, while most have not.9-11
Two studies have suggested that treatment of trichomoniasis with metronidazole in pregnancy is associated with an increased risk of preterm birth and low birth weight.12,13 In one of these, a multicentre trial in the USA, women with asymptomatic trichomoniasis treated with metronidazole were significantly more likely than those not treated to deliver a preterm infant.12 The second, a sub-analysis of a study in rural Uganda, showed that women who were treated for trichomoniasis were 2.5 times more likely to deliver a low-birth-weight infant compared with controls.13
In a planned secondary analysis we hypothesised, on the basis of these earlier studies, that women with trichomoniasis who were randomised to the study group given metronidazole (active against T. vaginalis) would have a higher rate of preterm and low-birth-weight infants than women randomised to placebo.
Materials and methods
HPTN 024 was a trial of antibiotics to reduce both mother-to-child transmission of HIV and preterm birth.14 The first women were enrolled in July 2001, and the last delivery occurred in August 2003. The study was conducted in four sub-Saharan African sites: Blantyre and Lilongwe, Malawi; Dar es Salaam, Tanzania; and Lusaka, Zambia. Enrolment required knowledge of HIV infection status. In three of the four sites, 1 HIV-uninfected woman was enrolled for every 5 infected women in order to mask HIV infection status. In Dar es Salaam, only HIV-infected women were enrolled. Women were enrolled between 20 and 24 weeks.
At enrolment, blood for syphilis testing and cervico-vaginal samples were obtained. Vaginal samples were analysed by microscopy in site laboratories for the presence of motile Trichomonas, candidiasis and bacterial vaginosis (through whiff test, vaginal pH, and presence of clue cells). Cotton-tipped applicators were also used to collect vaginal specimens for Gram staining, which were shipped to the University of Alabama at Birmingham for evaluation. For gonococcal culture, a cotton-tipped swab was placed in the endocervical canal and allowed to sit for at least 10 seconds. This swab was then used to inoculate a plate for gonococcal isolation. Chlamydia infection was detected by Chlamydia trachomatis antigen enzyme immunosorbent assay. Women were offered treatment for these infections at no cost, although treatment regimens differed across the sites.
Women were randomised to receive either antibiotics (metronidazole 250 mg and erythromycin 250 mg orally, both 3 times daily for 7 days) or placebo. Tests were repeated between 26 and 30 weeks' gestation for bacterial vaginosis, vaginal candidiasis and trichomoniasis. Women found to have any of these infections were treated, but not all women received treatment. With the onset of labour contractions and/or premature rupture of membranes (PROM), study participants initiated a second oral course of antibiotics consisting of metronidazole 250 mg orally and oral ampicillin 500 mg or placebo every 4 hours until delivery. These antibiotics were chosen because the organisms associated with clinical or sub-clinical chorioamnionitis (bacterial vaginosis-associated organisms such as Ureaplasma, Mycoplasma, Bacteroides, Mobiluncus and Gardnerella) are generally sensitive to one or more of these antibiotics.15
Because the study was conducted in four resource-limited settings and ultrasound was not generally available, it was not used to determine the estimated due date. Gestational age was determined by patient recall of the last menstrual period (LMP), corroborated with uterine size. If uterine size was not within 2 weeks of recall of the LMP, uterine size defined the obstetric gestational age. At delivery, each baby also had a modified Ballard examination to allow for a paediatric estimation of gestational age.16 If the Ballard examination and obstetric gestational age determination were consistent within 2 weeks, the obstetric gestational age was used; if not, the final study gestational age was based on the Ballard examination. Nurses at all locations were trained to determine both the obstetric and the paediatric gestational ages. Preterm birth was defined as birth occurring before 37 completed weeks of gestation, low birth weight as birth weight less than 2 500 g, and very low birth weight as birth weight less than 1 500 g. Results are presented independently for each of the methods. The analysis was restricted to singleton deliveries because twins have a higher baseline risk of preterm birth.17
Statistical analysis
We performed a secondary analysis using data from the HPTN 024 trial.11 Independent two-sample t-tests, Wilcoxon rank sum tests and two-sample chi-square tests were performed to compare means, medians, and proportions, respectively. Kaplan-Meier estimates were calculated and log rank tests were performed in time-to-event analysis of time to delivery following randomisation.
Results
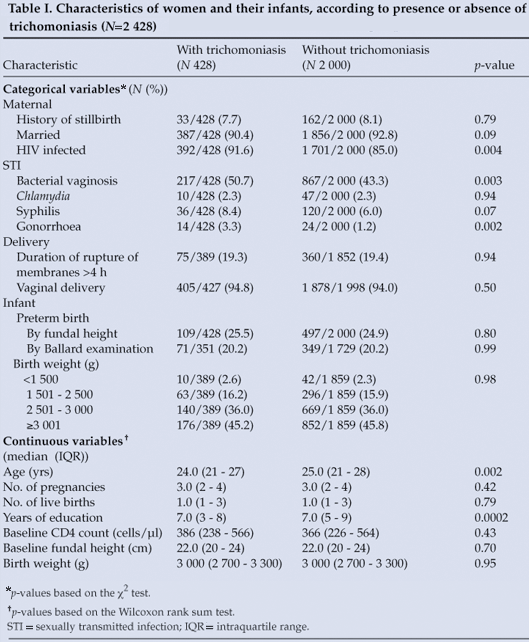
A total of 2 661 women were enrolled into HPTN 024 trial and randomised (86% of whom were HIV infected). Of these, 2 656 (almost 100%) had a baseline trichomoniasis result, but 188 (7%) were lost to follow-up before delivery, 32 (1%) delivered twins, 7 died before delivery, and 1 woman's delivery outcome was unknown. The study population therefore comprised 2 428 mother-infant pairs. Characteristics of the study population according to presence or absence of trichomoniasis at enrolment are set out in Table I. Compared with those without trichomoniasis, women with trichomoniasis were more likely to be HIV infected, to have bacterial vaginosis or gonorrhoea, to be younger, and to be slightly less educated (Table I).
Of the 428 women diagnosed with trichomoniasis, 231 (54%) were randomised to receive the study antibiotics and 197 (46%) were randomised to placebo (Table II and Fig. 1). There were no significant differences in baseline characteristics between the two groups. Women randomised to receive antibiotics were more likely to have resolution of trichomoniasis at their second visit than women randomised to placebo (p<0.0001). In the analysis by randomisation group, women randomised to the antibiotic arm were not more likely to deliver a preterm infant, whether the gestational age was based on fundal height obtained at randomisation (23.8% v. 27.4%; p=0.39) or on Ballard examination (20.6% v. 19.8%; p=0.84) or to deliver an infant with a lower mean birth weight (2 992 g v. 2 930 g; p=0.27) (Table II).
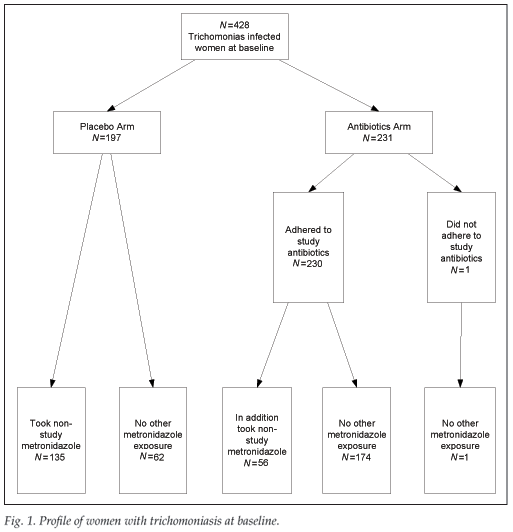
A second analysis was performed based on actual metronidazole use. Of the 428 women diagnosed with trichomoniasis, 365 (85%) received antibiotics active against trichomoniasis and 63 (17%) did not (Fig. 1). Women who received antibiotics active against trichomoniasis were more likely to have a Chlamydia infection and to have resolution of the trichomoniasis at their second visit compared with women who did not (p<0.001). Women who were diagnosed with trichomoniasis but did not receive antibiotics active against this infection were more likely to deliver a preterm infant when that assessment was based on symphysis-fundal height gestational age assessment (40% v. 23%; p=0.005), but not when the gestational age assessment was based on Ballard estimation (19% v. 20%; p=0.97). There were no differences in mean birth weights at delivery between women not receiving and those receiving treatment for trichomoniasis (2 972 g and 2 911 g, respectively) (Table III).
To examine the association of receipt of antibiotics and infant outcomes further among women with treated trichomoniasis, we examined time from randomisation to delivery for women with trichomoniasis who were treated with antibiotics compared with those who were not treated, since calculation of gestational age may be inaccurate without an ultrasound scan. There was no difference in the time from randomisation to delivery between those with treated and untreated trichomoniasis (log-rank p-value = 0.12) (data not shown).
Discussion
We found no differences in pregnancy outcomes among women diagnosed with trichomoniasis compared with women without trichomoniasis. Analysis of the treatment results of women who had trichomoniasis by both randomisation arm and actual-use analysis found no substantial differences in pregnancy outcomes. Women in the actual-use analysis who received metronidazole were more likely to have a preterm infant when gestational age was based on fundal height obtained at randomisation, but not when it was based on modified Ballard examination. In addition, there were no significant differences in infant birth weights between women with trichomoniasis according to randomisation arm or actual receipt of antibiotics. The time to delivery also did not differ according to randomisation arm or actual receipt of antibiotics.
A systematic review of well-conducted studies of pregnant women with either bacterial vaginosis or trichomoniasis receiving antibiotics in the second or third trimesters concluded that treatment of trichomoniasis in pregnancy may be harmful.18 This conclusion was primarily based on two studies. One showed that women with asymptomatic trichomoniasis treated with two 2 g doses of metronidazole 48 hours apart were more likely to have a preterm infant (relative risk (RR) 1.8, 95% confidence interval (CI) 1.2 - 2.7), but were not more likely to have a low-birth-weight infant (RR 1.4, 95% CI 0.9 - 2.1).12 The second study was a sub-analysis from a large community randomised trial in Uganda.13 This study found that pregnant women with trichomoniasis in the treatment arm (which included 2 g metronidazole) were 2.5 times as likely to deliver a low-birth-weight infant at 1 week of follow-up compared with women in the untreated arm. A major limitation of this study was its preferential follow-up of infants in the treatment arm; 84% of infants born to women in the treatment group were followed up within the first week, as opposed to only 67% of infants in the control group. In addition, there was no statistically significant difference in gestational age at birth between the control and treatment arms. Another observational study conducted in South Africa found no difference in mean birth weight or gestational age between infants born to women who were treated for trichomoniasis and those who were not.19
Our results are consistent with the findings in other studies that women who received antibiotic treatment for trichomoniasis are more likely to have resolution of their trichomoniasis. For example, trichomoniasis in our study was reduced by 68% in the group who received antibiotics active against trichomoniasis versus 21% in the placebo group (as previously reported). Women diagnosed with trichomoniasis in our population also were more likely to have other infections such as HIV infection, bacterial vaginosis, Chlamydia infection, and gonorrhoea than women without trichomoniasis. One limitation of our study is that a significant number of women randomised to the placebo arm in HPTN 024 were exposed to metronidazole. Because of this we also performed an actual-use analysis, for which results were similar to those of the intention-to-treat analysis. Another limitation of this study is in the imperfect gestational age dating using fundal height estimation and the modified Ballard examination. Since the modified Ballard examination can be performed only on women who deliver at a health centre, all of the women who were lost to follow-up, had a stillbirth, or delivered at home could not have a Ballard examination performed on their infant. The sample size for analyses including a modified Ballard examination was therefore diminished.
In conclusion, data regarding whether treatment of trichomoniasis in pregnancy is associated with adverse infant outcomes is limited by variation in the types of study, study size and even treatment regimens. In addition, some studies have examined treatment of asymptomatic trichomoniasis while others, such as ours, have examined antibiotic receipt by both asymptomatic and symptomatic subjects. Our results for sub-Saharan African women, mostly HIV-infected and with a high prevalence of trichomoniasis, do not indicate an increased risk of preterm birth according to both intention-to-treat and actual-use analyses. While this study was not designed to provide conclusive evidence of safety, we believe that our results, taken with other available evidence, do not suggest that treatment of trichomoniasis is associated with a substantially increased risk of preterm birth among HIV-infected African women.
This work was supported by the HIV Prevention Trials Network (HPTN) and sponsored by the US National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health, Department of Health and Human Services, through contract N01-AI-35173 with Family Health International; contract N01-AI-45200 with Fred Hutchinson Cancer Research Center; and subcontract N01-AI-35173-117/412 with Johns Hopkins University. In addition, this work was supported and sponsored by the National Institute of Allergy and Infectious Diseases, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institute on Drug Abuse, National Institute of Mental Health, and Office of AIDS Research, of the National Institutes of Health, US Department of Health and Human Services, Harvard University (U01-AI-480006), Johns Hopkins University (U01-AI-48005), and the University of Alabama at Birmingham (U01-AI-47972). Nevirapine (Viramune) for the study was provided by Boehringer Ingelheim Pharmaceuticals, Inc. The conclusions and opinions expressed in this paper are those of the authors and do not necessarily reflect those of the funding agencies and participating institutions.
HPTN 024 Team: Protocol Co-Chairs: Taha E Taha, MD, PhD (Johns Hopkins University Bloomberg School of Public Health); Robert Goldenberg, MD (University of Alabama at Birmingham); In-Country Co-Chairs/Investigators of Record: Newton Kumwenda, PhD, George Kafulafula, MB BS, FCOG (Blantyre, Malawi); Francis Martinson, MD, PhD (Lilongwe, Malawi); Gernard Msamanga, MD, ScD (Dar es Salaam, Tanzania); Moses Sinkala, MD, MPH, Jeffrey Stringer, MD (Lusaka, Zambia); US Co-Chairs: Irving Hoffman, PA, MPH (University of North Carolina, Chapel Hill); Wafaie Fawzi, MD, DrPH (Harvard School of Public Health ); In-Country Investigators, Consultants and Key Site Personnel: Robin Broadhead, MB BS, FRCP, George Liomba, MB BS, FRCPath, Johnstone Kumwenda, MB ChB, MRCP, Tsedal Mebrahtu, ScM, Pauline Katunda, MHS, Maysoon Dahab, MHS (Blantyre, Malawi); Peter Kazembe, MB ChB, David Chilongozi, CO, MPH, Charles Chasela, CO, MPH, George Joaki, MD, Willard Dzinyemba, Sam Kamanga (Lilongwe, Malawi); Eligius Lyamuya, MD, PhD, Charles Kilewo, MD, MMed, Karim Manji, MD, MMed, Sylvia Kaaya, MD, MS, Said Aboud, MD, MMed, Muhsin Sheriff, MD, MPH, Elmar Saathoff, PhD, Priya Satow, MPH, Illuminata Ballonzi, SRN, Gretchen Antelman, ScD, Edgar Basheka, BPharm (Dar es Salaam, Tanzania); Victor Mudenda, MD, Christine Kaseba, MD, Maureen Njobvu, MD, Makungu Kabaso, MD, Muzala Kapina, MD, Anthony Yeta, MD, Seraphine Kaminsa, MD, MPH, Constantine Malama, MD, Dara Potter, MBA, Maclean Ukwimi, RN, Alison Taylor, BSc, Patrick Chipaila, MSc, Bernice Mwale, BPharm (Lusaka, Zambia); US Investigators, Consultants and Key Site Personnel: Priya Joshi, BS, Ada Cachafeiro, BS, Shermalyn Greene, PhD, Marker Turner, BS, Melissa Kerkau, BS, Paul Alabanza, BS, Amy James, BS, Som Siharath, BS, Tiffany Tribull, MS (UNC-CH); Saidi Kapiga, MD, ScD, George Seage, PhD (HSPH); Sten Vermund, MD, PhD, William Andrews, PhD, MD, Deedee Lyon, BS, MT (ASCP) (UAB); NIAID Medical Officer: Samuel Adeniyi-Jones, MD; NICHD Medical Officer: Jennifer S Read, MD, MS, MPH, DTM&H; Protocol Pharmacologist: Scharla Estep, RPh, MS; Protocol Statisticians: Elizabeth R Brown, ScD, Thomas R Fleming, PhD, Anthony Mwatha, MS, Lei Wang, PhD, Deborah Donnell, PhD, Ying Q Chen, PhD; Protocol Virologist: Susan Fiscus, PhD; Protocol Operations Coordinator: Lynda Emel, PhD; Data Coordinators: Debra J Lands, EdM, Ceceilia J Dominique; Systems Analyst Programmers: Alice H Fisher, BA, Martha Doyle; Protocol Specialist: Megan Valentine, PA-C, MS.
References
1. Hack M, Fanaroff AA. Outcomes of extremely premature infants: a perinatal dilemma. N Engl J Med 1993; 329: 1649-1650. [ Links ]
2. Kulmala T, Vaahtera M, Ndekha M, et al. The importance of preterm births for peri- and neonatal mortality in rural Malawi. Paediatr Perinatal Epidemiol 2000; 14(3): 219-226. [ Links ]
3. Hillier SL, Nugent RP, Eschenbach DA, et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The Vaginal Infections and Prematurity Study Group. N Engl J Med 1995; 333(26): 1737-1742. [ Links ]
4. Gravett M, Nelson HP, DeRouen T, Critchlow C, Eschenbach DA, Holmes KK. Independent associations of bacterial vaginosis and Chlamydia trachomatis infection with adverse pregnancy outcome. JAMA 1986; 256(14): 1899-1903. [ Links ]
5. Cotch MF, Pastorek JG, Nugent RP, et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. The Vaginal Infections and Prematurity Study Group. Sex Transm Dis 1997; 24(6): 353-350. [ Links ]
6. Buve A, Weiss HA, Laga M, et al. The epidemiology of trichomoniasis in women in four African cities. AIDS 2001; 15: Suppl 4, S89-S96. [ Links ]
7. Price MA, Zimba D, Hoffman IF, Kaydos-Daniels SC, Miller WC, Martinson F. Addition of treatment for trichomoniasis to syndromic management of urethritis in Malawi: a randomized clinical trial. Sex Transm Infect 2003; 30(6): 516-522. [ Links ]
8. Watson-Jones D, Mugeye K, Mayaud P, Ndeki L, Todd J, Mosha F. High prevalence of trichomoniasis in rural men in Mwanza, Tanzania: results from a population based study. Sex Transm Infect 2000; 76: 355-362. [ Links ]
9. Hauth JC, Goldenberg RL, Andrews WW, DuBard MB, Copper RL. Reduced incidence of preterm delivery with metronidazole and erythromycin in women with bacterial vaginosis. N Engl J Med 1995; 333(26): 1732-1736. [ Links ]
10. Lamont RF. Can antibiotics prevent preterm birth – the pro and con debate. BJOG 2005; 112: Suppl 1, 67-73. [ Links ]
11. Hutzal CE, Boyle EM, Kenyon SL, et al. Use of antibiotics for the treatment of preterm parturition and prevention of neonatal morbidity: a metaanalysis. Am J Obstet Gynecol 2008; 199(6): 620.E1-8. [ Links ]
12. Klebanoff MA, Carey JC, Hauth JC, et al. Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic Trichomonas vaginalis infection. N Engl J Med 2001; 345: 487-493. [ Links ]
13. Kigozi GG, Brahmbhatt H, Wabwire-Mangen F, et al. Treatment of Trichomonas in pregnancy and adverse outcomes in pregnancy: a subanalysis of a randomized trial in Rakai, Uganda. Am J Obstet Gynecol 2003; 189(5): 1398-1400. [ Links ]
14. Taha TE, Brown ER, Hoffman IF, et al. A phase III clinical trial of antibiotics to reduce chorioamnionitis-related perinatal HIV-1 transmission. AIDS 2006; 20(9): 1313-1321. [ Links ]
15. Goldstein EJC, Citron DM, Cherubin CE, Hillier SL. Comparative susceptibility of the Bacteroides fragilis group species and other anaerobic bacteria to meropenem, imipenem, piperacillin, cefoxitin, ampicillin/sulbactam, clindamycin and metronidazole. J Antimicrob Chemother 1993; 31(3): 363-372. [ Links ]
16. Ballard JL, Novak KK, Driver MA. A simplified score for assessment of fetal maturity of newly born infants. J Pediatr 1979; 95(5, Pt I): 769-774. [ Links ]
17. Herruzo AJ, Martinez L, Biel E, Robles R, Rosales MA, Miranda JA. Perinatal morbidity and mortality in twin pregnancies. Int J Gynaecol Obstet 1991; 36(1): 17-22. [ Links ]
18. Okun N, Gronau KA, Hannah ME. Antibiotics for bacterial vaginosis or Trichomonas vaginalis in pregnancy: A systematic review. Obstet Gynecol 2005; 105: 857-868. [ Links ]
19. Ross SM, Middelkoop A. Trichomonas infection in pregnancy: does it affect perinatal outcome? S Afr Med J 1983; 63: 566-567. [ Links ]
Accepted 4 September 2009.
* Corresponding author: E Stringer (eli@uab.edu)














