Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.100 no.1 Pretoria Jan. 2010
ORIGINAL ARTICLES
Helicobacter pylori: Prevalence and antibiotic susceptibility among Kenyans
Andrew Nyerere Kimang'aI,*; Gunturu RevathiII; Samuel KariukiV; Shahin SayedIII; Smita DevaniIV
IBEd, MSc, Medical Microbiology Subdepartment, Jomo Kenyatta University of Agriculture and Technology, Nairobi, Kenya
IIMB BS, MD, Department of Pathology, Aga Khan University Hospital, Nairobi
IIIMB ChB, MMed, Department of Pathology, Aga Khan University Hospital, Nairobi
IVMB ChB, MRCP, Department of Internal Medicine, Aga Khan University Hospital
VBVSc, MSc, PhD, Center for Microbiology Research, Kenya Medical Research Institute, Nairobi
ABSTRACT
BACKGROUND: Helicobacter pylori infection in Kenya is staggeringly high. Evidence links infection of the gastric mucosa by H. pylori with subsequent development of gastric pathologies.
AIM: We investigated the prevalence of H. pylori in dyspeptic patients, its relationship with gastric pathologies, and associated antibiotic susceptibility profiles, and compared two media to find the appropriate medium that enhances growth and expedites culture and isolation.
METHODS: Rapid urease and histological tests were used to screen for H. pylori. Culture was performed to test sensitivity and evaluate media. Selective and nutritional supplements were added to culture media (Colombia blood agar and brain-heart infusion agar) for growth enhancement. E-test strips for metronidazole, amoxicillin and clarithromycin were used for susceptibility testing.
RESULTS: The prevalence of H. pylori infection in children was 73.3%, and 54.8% in adults. All the H. pylori investigated in this study were largely sensitive to clarithromycin (100%, minimum inhibiting concentration (MIC) <2 µg/ml), amoxicillin (100%, MIC <2 µg/ml) and metronidazole (95.4%, MIC <8 µg/ml). There was, however, occasional resistance to metronidazole (4.6%, MIC >8 µg/ml). Both Colombia blood and brain-heart infusion agar, with the supplements, effectively supported H. pylori growth. Growth was achieved in an average of 36 hours for primary isolations and 24 hours for subcultures.
CONCLUSION: The media described here reduce the time required to culture and isolate bacteria and perform susceptibility testing. Despite the high prevalence of H. pylori infection, the associated pathology is low and does not parallel H. pylori prevalence in the population.
Increasing evidence links infection of the gastric mucosa by Helicobacter pylori with the subsequent development of gastric pathologies. The incidence of H. pylori infection in Kenya is staggeringly high. An investigation into the prevalence of H. pylori in patients with dyspepsia and asymptomatic controls from the same population, showed high prevalence levels in both groups.1 Seropositivity for H. pylori was found in 98 (71%) symptomatic patients and in 70 (51%) asymptomatic participants. McFarlane et al. linked infection of the gastric mucosa by H. pylori in Kenya with the subsequent development of gastric atrophy.2 H. pylori prevalence in Kenya shows no difference between HIV-positive and -negative subjects.3. However, data on H. pylori antibiotic sensitivity profiles in Kenya are scarce because of lack of reliable and affordable media on which isolation and susceptibility testing of H. pylori can be performed.4-7 Lack of information on the susceptibility patterns of H. pylori strains implicated in infections leads to poor management, resulting in treatment failures. Resistance to metronidazole and clarithromycin has been reported to be highly prevalent and more common elsewhere.8 It is therefore important to find a medium that easily supports the growth of H. pylori and allows prompt susceptibility testing. We also investigated the prevalence of H. pylori infection in Kenya and associated disease outcomes.
Materials and methods
Patient population
We recruited 696 patients who had presented with dyspepsia at the gastroenterology clinic of the Aga Khan University Hospital, Nairobi, and who had been referred for endoscopy. They were all screened for H. pylori by the rapid urease and histological tests. Of the 696 patients, 70 (10%), who had positive stool antigen test prior to endoscopy and who had no history of antibiotic use in the 3 months preceding their visit to the clinic, were selected for H. pylori culture. The study was approved by the Aga Khan University Hospital Research and Ethics Committee. Biopsies (2 each from the antrum and corpus) were taken, submerged in 5% brain-heart-infusion agar (BHIA), enriched with 5% fetal bovine serum (FBS) and kept at 4ºC till processing. All biopsies were processed within 3 hours of extraction.
Culture media preparation
Inactivation of fetal bovine serum
FBS to be used for culture media preparation was completely thawed at room temperature, transferred to a water bath at 56°C and stirred manually every 10 minutes. When the FBS reached 56°C (indirectly measured by the temperature of the water bath), it was incubated for 30 minutes, cooled to room temperature, dispensed in aliquots of 35 ml into tubes labeled 'Inactivated FBS', and stored at -20°C. Sterility control was performed by incubating an aliquot of the FBS at 37°C for 48 hours.
H. pylori selective supplement (Dent) and nutritional supplement (Vitox)
To a bottle of Dent, 2 ml of sterile distilled water was added and mixed gently. The solution was used the same day. Vitox was prepared by mixing the provided solvent and the Vitox powder. All were prepared according to the manufacturers' instructions.
Transport medium (5% BHIA + 5% FBS)
The above is the semi-solid agar for transportation of gastric biopsies. Five grams of BHIA was weighed, suspended in 95 ml of sterile distilled water, dissolved by boiling, and subsequently autoclaved at 121°C for 15 minutes. After autoclaving, the medium was cooled to 45°C in a water-bath and gently mixed with a 5 ml aliquot of the inactivated FBS. Aliquots of 500 µl were dispensed into 1.5 ml tubes and stored at 4°C in a refrigerator until use. The transport medium was used within 1 week from the preparation date. A sterility control was done on one tube from the preparation under aerobic and micro-aerophilic conditions, at 37°C for 24 hours.
Non-selective culture media: Colombia blood agar (CBA) + 7% FBS and BHIA + 7% FBS plates (CBA + 7% FBS and BHIA + 7% FBS)
CBA (19.5±0.1 g) and BHIA (23.5±0.1 g) (Oxoid, UK) were each weighed and put in separate 500 ml bottles. Both media were suspended in 500±0.5 ml sterile distilled water and fully dissolved by boiling. These bottles were labeled 'CBA + 7% FBS' and 'BHIA + 7% FBS' respectively and autoclaved at 121°C for 15 minutes. After autoclaving, the media were cooled to 45°C in a water-bath and gently mixed with 35 ml aliquots of the inactivated FBS and the Vitox supplement. The media were poured (approximately 25 ml per plate), left to solidify, and dried at room temperature for 2 hours before being stored at 8°C in a refrigerator until use. The plates were used within 1 week from the preparation date. Sterility control on 2 plates from each pack of 20 plates was done at 37°C for 24 hours, 1 under aerobic and 1 under micro-aerophilic conditions.
Selective culture media: CBA + 7% FBS + Dent, and BHI agar + 7% FBS + Dent plates (CBAD + 7% FBS and BHIAD + 7% FBS)
(CBAD = Colombia blood agar with Dent selective supplement containing vancomycin, trimethoprim, cefsulodin and amphotericin B; BHIAD = brain-heart-infusion agar with Dent selective supplement containing vancomycin, trimethoprim, cefsulodin and amphotericin B.)
CBA (19.5±0.1 g) and BHIA (23.5±0.1 g) (Oxoid, UK) were each weighed and put in separate 500 ml bottles. Both media were suspended in 500±0.5 ml of sterile distilled water and were fully dissolved by boiling. These bottles were labelled 'CBAD + 7% FBS' and 'BHIAD + 7% FBS' respectively, autoclaved at 121°C for 15 minutes, cooled down to 45°C in a water-bath, and gently mixed with the inactivated FBS; thereafter, Dent and Vitox were added and mixed by gently rolling the bottle. The media were poured (approximately 25 ml per plate), left to solidify, and dried at room temperature for 2 hours before being stored at 8°C in a refrigerator until use. The plates were used within 1 week from the preparation day. Sterility control at 37°C for 24 hours on two plates from each pack of 20 plates was done; 1 under aerobic and 1 under micro-aerophilic conditions.
H. pylori growth
The biopsies were transferred by sterile 1 000 µl pipette to clean sterile tubes, where they were completely crushed using the pipette tip; 200 µl of BHI broth, enriched with 5% FBS, was added and uniformly mixed. Next, 100 µl of the ruptured and homogenised material was aseptically transferred to each of the appropriately labelled plates, one selective and the other non-selective, using a disposable 10 µl sterile inoculating loop. The inoculum was aseptically spread on the surface of the plates. Inverted, the plates were placed in a 2.5 l jar. A Campy Micro-aerophilic kit was added, and the jar immediately closed and incubated undisturbed at 37°C for 3 days. After 3 days, the plates were removed and visually inspected. When the colonies appeared too small, a new Campy Micro-aerophilic kit was placed in the 2.5 l jar and the plates re-incubated immediately for a further 2 days. If growth was not satisfactory after 5 days, further incubation was done up to 7 days with a new Campy Micro-aerophilic kit until the colonies were well visible. H. pylori identification was confirmed by colony morphology, Gram-stain, and oxidase, urease and catalase positivity. Old cultures were a mixture of short rods and coccoids, while young cultures were curved rods. Subcultures were done on antibiotic-free media. Pure culture was harvested by transferring 200 µl phosphate-buffered saline (PBS) onto the plate. The pure bacteria growth on the plate was emulsified in the PBS using a sterile inoculating loop. The suspension was then transferred to cryotubes containing 500 µl BHI broth enriched with 20% glycerol and 5% FBS, and stored at -80°C.
H. pylori sensitivity testing
A frozen vial of the correctly identified H. pylori was thawed at room temperature and mixed by pippeting up and down with a micropipette. This sample was used for subculture. Young, pure cultures were harvested by transferring 200 µl PBS onto the culture plate and the bacteria emulsified into a suspension using a sterile inoculating loop. The suspension was collected into a sterile tube and a McFarland's turbidity standard no. 3 made for use in susceptibility testing. The inoculum was aseptically spread over the entire portion of the surface of the sensitivity test plate, and allowed to stand at room temperature for 10 minutes before the antibiotic E-test strips for clarithromycin (0.016-256); metronidazole (0.002-32) and amoxicillin (0.016-256) were applied. Inverted inoculated plates were then placed into the jar. One envelope of Campy Micro-aerophilic gas kit was put into the 2.5 l jar following the manufacturer's instructions. The jar was incubated at 37°C for 24 hours, and then removed and inspected for satisfactory H. pylori growth. If the growth were insufficient, the plates were immediately put back into the jar with a new CampyGen Micro-aerophilic gas kit, and incubation continued at 37°C for a further 12 hours. When growth was satisfactory, the minimum inhibiting concentration (MIC) for each antibiotic was read as recommended by the National Committee on Clinical Laboratory Standards/Clinical and Laboratory Standards Institute (NCCLS/CLSI) standards.
Results
Of the 696 patients with dyspepsia who were screened, 66.1% were adults and 33.9% were children. The prevalence of H. pylori infection in the adult segment was 54.8%; and 73% in children (Fig. 1). Culture was attempted on 70 biopsies; of these, 65 (92.8%) were successful.

Prevalence of H. pylori infection was 65% in African immigrants, 25% in Asian immigrants, 56% in rural Kenyan Africans, 62% in urban Kenyan Africans, and 58% among urban Kenyan Asians. Gastro-esophageal reflux disease (GERD) accounted for 201 (28.9%) of the cases, gastric polyps 3 (0.4%), atrophic mucosa 4 (0.6%), gastric cancer 6 (0.9%), gastritis 452 (64.9%), duodenal ulcer 12 (1.7%), and gastric ulcer 18 (2.6%) (Fig. 2). GERD was more common in Kenyan female patients (56 - 65.6%) than males.
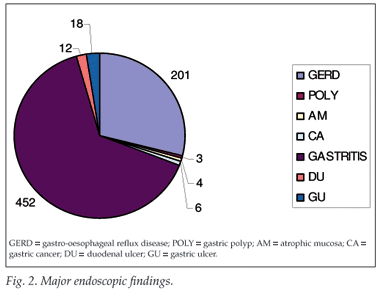
Gastric and duodenal ulcers occur with equal frequency in all racial groups. Despite a high prevalence (67.5%) of H. pylori in Kenya, the complicated disease outcomes (such as gastric cancer) are relatively low in this series (Table I).

Results of susceptibility testing for metronidazole (MZ), amoxicillin (AX) and clarithromycin (CL) and the total number of H. pylori bacilli susceptible at different concentration of these drugs are summarised in Fig. 3. MIC levels of <8 µg/ml for MZ and AX, and <2 µg/ml for CL, were interpreted as sensitive.

All the H. pylori that we investigated were largely sensitive to clarithromycin (100%), amoxicillin (100%) and metronidazole (95.4%). However, there was occasional resistance to metronidazole (4.6%). The 3 antibiotics showed no significant difference in activity (mean±SD: MZ 5±8.6, CL 5±7.3, AX 5±5.3, p=1). Both CBA and BHIA effectively supported H. pylori growth achieved in an average of 36 hours for primary isolations and 24 hours for subcultures (data not shown).
Discussion
Prevalence of H. pylori was found to be 67.5% in all age groups (73.3% in children and 54.8% in adults), similar to the findings of Shmuely et al,1 who documented 60 - 73% in all age groups in dyspeptic patients. However, this finding differs with studies in other African countries which found H. pylori prevalence levels of >90%.9-13 Gastritis accounted for the largest proportion (64.9%) of the H. pylori-associated pathologies, as found elsewhere in Africa.9 It has been noted that, despite the prevalence of a virulent strain and high prevalence of H. pylori, the associated pathology (duodenal ulcer, gastric ulcer and gastric cancer) is variable and often low-incidence, and its distribution in sub-Saharan Africa does not parallel H. pylori prevalence in the population.14,15 This phenomenon was strongly supported by the findings of our study, where atrophic mucosa represented 0.6%, gastric cancer 0.9%, duodenal ulcer 1.7%, and gastric ulcer 2.6% of all observed pathologies, despite the high prevalence (67.5%) of H. pylori infection, which implies that, in this decade, the old status quo still stands.
We found no difference in the distribution of H. pylori pathologies among the different ethnic inhabitants of Kenya. In 2001, Segal et al.14 reported that, despite the presence of virulent strains in Africa, associated pathologies are not in tandem. He suggested that host immune response may be a factor in protecting against development of gastric cancer. Our findings seem to suggest otherwise. If host immune response were a factor, we should see a varied distribution of pathologies in our study population of different ethnic groups. Therefore, we suggest that the development of significant clinical pathology in H. pylori infection depends on a combination of complex factors, possibly host genetics, environment, pathogens and age of the host.
All the H. pylori investigated in this study were largely sensitive to clarithromycin (100%), amoxicillin (100%) and metronidazole (95.4%). There was, however, occasional resistance to metronidazole (4.6%). The 3 antibiotics showed no significant difference in activity (mean±SD: MZ 5±8.6, CL 5±7.3, AX 5±5.3, p=1). Susceptibility rates in Cameroon were found to be 55.3% for clarithromycin, 14.4% for amoxicillin and 6.8% for metronidazole.13 The documented resistance of amoxicillin in Africa signifies the emergence of H. pylori strains resistant to this key component in H. pylori treatment protocols. Up to the year 2000, amoxicillin-resistant H. pylori strains had rarely been detected, with a total of only 14 reported.16 Significantly, complete loss of the resistant phenotype was observed after these strains were stored at -80°C, owing to the process of freezing and thawing. Only one amoxicillin-resistant H. pylori strain had been isolated, in the Netherlands, in which amoxicillin resistance remained stable after repeated cycles of freezing and culture.17 The MIC for this strain was 8 µg/ml, which is relatively low. A survey is therefore necessary to confirm the presence of amoxicillin-resistant H. pylori strains in Africa. Amoxicillin-resistant H. pylori strains in circulation are a threat to public health, as this resistance trait may be transferable to amoxicillin-susceptible strains,17 presumably owing to DNA exchange by transformation or a conjugation-like mechanism.18 Our study, however, shows a high susceptibility to amoxicillin among H. pylori strains.
The media we described support H. pylori growth in an average of 36 hours for primary isolations and 24 hours for subcultures, with an isolation rate of 92.8% (65/70) whereas an isolation rate of 47% (31/66) in Kenya was achieved in growth using Modified Thayer-Martin agar after 4 days of incubation.4 The medium used in this study is therefore considered superior and can be used for enhanced cultivation of H. pylori and susceptibility testing.
Lastly, the great diversity of ethnic and racial groups in Kenya, and the high prevalence of H. pylori but the low prevalence of gastric carcinoma, call for closer examination of H. pylori in different regions and ethnic groups within Kenya as well as within surrounding regions. This investigation can provide new data regarding both the diversity of H. pylori as well as the co-migration of H. pylori and its human hosts.
This study was funded by the Deutscher Akademischer Austausch Dienst (German Academic Exchange Service) (DAAD), and was supported by the Aga Khan University Hospital and Jomo Kenyatta University of Agriculture and Technology, Nairobi, Kenya.
References
1. Shmuely H, Samson O, Passaro DJ, et al. Dyspepsia symptoms and Helicobacter pylori infection, Nakuru, Kenya. Emerg Infect Dis 2003; 9(9): 1103-1106. [ Links ]
2. McFarlane GA, Wyatt J, Forman D, et al. Trends over time in Helicobacter pylori gastritis in Kenya. Eur J Gastroenterol Hepatol 2000; 12: 617-621. [ Links ]
3. AliMohamed F, Lule GN, Nyong'o A, Bwayo J, Rana F. Prevalence of Helicobacter pylori and endoscopic findings in HIV seropositive patients with upper gastrointestinal tract symptoms at Kenyatta National Hospital, Nairobi. East Afr Med J 2002; 79(5): 225. [ Links ]
4. Sang FC, Lule GN, Ogutu EO. Evaluation of culture media and antimicrobial susceptibility of Helicobacter pylori. East Afr Med J 1991; 68(11): 865-868. [ Links ]
5. Pellicano R, Smedile A, Ponzetto A, et al. How accurate is the culture of Helicobacter pylori in a clinical setting? An appraisal. Panminerva Med 2005; 47(3): 191-194. [ Links ]
6. Graud FM, Lehn N, Lind T, et al. Antimicrobial susceptibility testing of Helicobacter pylori in a large multicenter trial: the MACH 2 Study Antimicrob Agents Chemother 1999; 43(11): 2747-2752. [ Links ]
7. Chomvarin C, Kulsantiwong P, Chantrakooptungool YCS, Kanjanahareutai S. Comparison of media and antibiotic supplements for isolation of Helicobacter pylori from gastric biopsies. Southeast Asian J Trop Med Public Health 2006; 37(6): 1163-1169. [ Links ]
8. Yuen B, Zbinden R, Fried M, Bauerfeind P, Bernardi M. Cultural recovery and determination of antimicrobial susceptibility in Helicobacter pylori by using commercial transport and isolation media. Infection 2005; 33: 77-81. [ Links ]
9. Carrilho C, Modcoicar P, Cunha L, et al. Prevalence of Helicobacter pylori infection, chronic gastritis, and intestinal metaplasia in Mozambican dyspeptic patients. Virchows Archiv 2009; 454(2): 153-160. [ Links ]
10. Fritz EL, Slavik T, Delport W, Olivier B, van der Merwe SW. Incidence of Helicobacter felis and the effect of coinfection with Helicobacter pylori on the gastric mucosa in the African population. J Clin Microbiol 2006; 44(5): 1692-1696. [ Links ]
11. Newton R, Ziegler JL, Casabonne D, et al. Helicobacter pylori and cancer among adults in Uganda. Infect Agent Cancer 2006; 1: 5. http://www.infectagentscancer.com/content/1/1/5 (accessed 14 April 2009). [ Links ]
12. Asrat D, Nilsson I, Mengistu Y, et al. Prevalence of Helicobacter pylori vacA and cagA genotypes in Ethiopian dyspeptic patients. J Clin Microbiol 2004; 42(6): 2682-2684. [ Links ]
13. Ndip RN, Takang M, Alertia E, et al. Helicobacter pylori isolates recovered from gastric biopsies of patients with gastro-duodenal pathologies in Cameroon: current status of antibiogram. Trop Med Int Health 2008; 13 (6): 848-854. [ Links ]
14. Segal I, Ally R, Mitchell H. Helicobacter pylori – an African perspective. Q J Med 2001; 94: 561-565. [ Links ]
15. Holcombe C. Helicobacter pylori: the African enigma. Gut 1992; 33(4): 429-431. [ Links ]
16. Dore MP, Sepulveda AR, Mura I, Realdi G, Osato MS, Graham DY. Explanation for variability of omeprazole amoxicillin therapy? Tolerance of H. pylori to amoxicillin. Gastroenterology 1997; 112: A105. [ Links ]
17. Van Zwet AA, Vandenbrouke-Grauls CMJE, Thijs JC, van der Wouden EJ, Gerrits MM, Kusters JG. Stable amoxicillin resistance in Helicobacter pylori. Lancet 1998; 352: 1595. [ Links ]
18. Kuipers EJ, Israel DA, Kusters JG, Blaser MJ. Evidence for a conjugation-like mechanism of DNA transfer in Helicobacter pylori. J Bacteriol 1998; 180: 2901-2905.22. [ Links ]
Accepted 24 August 2009.
* Corresponding author: A Kimang'a (kinyerere@yahoo.com)














