Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.100 no.1 Pretoria Jan. 2010
ORIGINAL ARTICLES
Marked susceptibility of South African Helicobacter pylori strains to ciprofloxacin and amoxicillin: Clinical implications
Nicoline F TanihI; Benjamin I OkeleyeII; Nathan NaidooIV; Anna M ClarkeIII; Noxolo MkwetshanaIII; Ezekiel GreenI; Lucy M NdipV; Roland N Ndip*,III,V
IMSc, Department of Biochemistry and Microbiology, University of Fort Hare, Alice, E Cape
IIBSc (Hons), Department of Biochemistry and Microbiology, University of Fort Hare, Alice, E Cape
IIIPhD, Department of Biochemistry and Microbiology, University of Fort Hare, Alice, E Cape
IVMB ChB, FCP (SA), Department of Gastroenterology, Livingstone Hospital, Port Elizabeth, E Cape
VPhD, Department of Biochemistry and Microbiology, Faculty of Science, University of Buea, Buea, Cameroon
ABSTRACT
OBJECTIVES: Helicobacter pylori-associated infection is common in South Africa, as in other developing countries. Antibiotic resistance is recognised as a major cause of treatment failure. We studied the susceptibility and resistance patterns of H. pylori to guide empiric treatment and prevent the emergence of resistance.
METHODS: Two hundred H. pylori strains obtained from gastric biopsies of patients presenting with gastric-related morbidities attending Livingstone Hospital, Port Elizabeth, were evaluated for their susceptibility to seven antibiotics – metronidazole, clarithromycin, tetracycline, amoxicillin, gentamicin, ciprofloxacin and erythromycin. H. pylori was isolated following standard microbiology procedures, and susceptibility determined using the Kirby-Bauer disc diffusion and agar dilution methods. Comparisons of antimicrobial resistance rates with sex of the patients were determined using the chi-square test; a p-value of <0.05 was considered significant.
RESULTS: Marked susceptibility was observed for ciprofloxacin (100%) and amoxicillin (97.5%), and good activity for clarithromycin (80%) and gentamicin (72.5%). However, marked resistance (95.5%) was observed for metronidazole. The minimal inhibitory concentration (MIC) ranged from 0.0625 µg/ml to 8 µg/ml. The lowest MIC, with a range of 0.0625 - 1 µg/ml, was recorded for ciprofloxacin, while the highest (5 - 8 µg/ml) was noted for gentamicin.
CONCLUSION: Multidrug resistance was commonly encountered – a finding of clinical significance that calls for continuous surveillance of antibiograms to guide empiric treatment. We advocate the inclusion of ciprofloxacin in the treatment regimen of H. pylori infection in our study environment.
Helicobacter pylori is the principal species of the genus Helicobacter that inhabits the gastric mucosa of the human stomach. It chronically infects billions of people worldwide1,2 and causes one of the most frequent chronic bacterial infections, involving more than 50% of the world's population.3 H. pylori is one of the most genetically diverse of bacterial species, and is a major cause of at least 90% of duodenal ulcers, 70% of gastric ulcers, non-ulcer dyspepsia, gastro-oesophageal reflux disease, adenocarcinoma of the distal stomach and mucosa-associated lymphoid tissue (MALT) lymphoma in many societies.4
H. pylori infection presents a unique therapeutic challenge. Determining the optimal treatment is difficult because the organism lives in an environment not easily accessible to many medications. Several antibiotic agents are used worldwide to treat these infections; despite this range of antibiotics, the problem of drug resistance to most of them is well established.2,5
Reliable treatment of H. pylori infection is difficult, and successful regimens generally require two or more antibiotics coupled with a proton pump inhibitor.6 Eradication of the organism is the first therapeutic approach and constitutes a reliable long-term prophylaxis of peptic ulcer relapse, accelerating ulcer healing and reducing the rate of ulcer complications.4 However, the prevalence of multidrug-resistant strains, especially in developing countries,7 makes it obligatory to perform culture and antibiotic sensitivity for patients with persistent infection after failure of initial or repeated treatment.1
Antibiotic resistance is a major cause of treatment failure. Primary resistance against clarithromycin and metronidazole is common in many countries,5,8 while resistance to different antibiotics used as first-line treatment varies between countries and communities, and may change with time and geographical location.1 In South Africa, the regimen for treating H. pylori infection comprises amoxicillin, metronidazole, clarithromycin and doxycycline (personal communication) and theoretically should demonstrate 85 - 95% efficacy.9
We recently identified H. pylori isolates from patients presenting with gastric diseases in the Eastern Cape. The organism is associated with substantial morbidity and mortality.2 An increase in treatment failure is emerging because of growing resistance to currently used antibiotics and the fact that the antibiotic susceptibility patterns of micro-organisms change with time and geographical location.1 Consequently, it is imperative to evaluate current susceptibility/resistance patterns of H. pylori strains in the Eastern Cape province in particular and South Africa in general, especially as existing studies are few and outdated,10,11 to guide empirical treatment and prevent the emergence of further resistance. We investigated the susceptibility and resistance patterns of these isolates to seven different antibiotics.
Methods
Bacterial strains
Two hundred H. pylori strains were isolated from patients suffering from gastric diseases and referred to Livingstone Hospital, Port Elizabeth, for endoscopy. Biopsies were taken after informed consent. The study was approved by the institutional review board of the University of Fort Hare and the Eastern Cape Department of Health (Protocol number EcDoH-Res 0002).
The organism was isolated and identified as previously reported.1 Biopsies were homogenised under aseptic conditions in 0.2 g/l of cysteine and 20% glycerol in brain-heart infusion broth and a loop full-plated primarily on freshly prepared Columbia agar base (Oxoid, Basingstoke, England) supplemented with 7% sheep's blood (Oxoid, England) and Skirrow's supplement (Oxoid, England); trimethoprim (2.5 mg), vancomycin (5 mg), cefsulodin (2.5 mg), and amphotericin (2.5 mg) were also added to the medium. All plates were incubated at 37ºC for 3 - 5 days under micro-aerophilic conditions (5 - 6% O2, 10% CO2, 80 - 85% N2) (Anaerocult, Basingstoke, England). Isolates were identified based on colony morphology and positive oxidase, urease and catalase tests. A reference strain of H. pylori (NCTC 11638) was included as a positive control. Confirmed isolates were suspended in 20% glycerol and stored at –80ºC in a freezer (Sanyo, Japan) for future experiments.
Antibiotic susceptibility testing
Susceptibility testing to determine zones of inhibition and minimum inhibitory concentration (MIC) was by the disk diffusion (Kirby-Bauer) technique and agar dilution methods respectively, which conform to the standard of the Clinical and Laboratory Standard Institute (CLSI)12 with little modification.1 Antibiotics usually indicated for triple therapy, namely metronidazole (5 µg), clarithromycin (15 µg), tetracycline (10 µg), amoxicillin (10 µg), gentamicin (10 µg), ciprofloxacin (5 µg) and erythromycin (10 µg) (Mast Diagnostics, Bootle, England), were used. This selection was based on the current treatment regimen in South Africa (personal communication) and elsewhere.6 Brain-heart infusion (BHI) agar (Oxoid, England) containing 7% horse blood and H. pylori-selective supplement (Oxoid, England) was used. H. pylori control strain NCTC 11638 was included in all the experiments to determine susceptibility or resistance.1 The zones of inhibition were interpreted as previously reported.1,13
For the MIC values, susceptibility for clarithromycin was interpreted according to the CLSI guideline.12 Since the CLSI has not designated breakpoints for H. pylori for all the antibiotics commonly used for treatment, the resistant breakpoint used for metronidazole, gentamicin and erythromycin was ≥8 µg/ml; ≥ 2 µg/ml was used for tetracycline, >1.0 µg/ml for clarithromycin, and ≥1.0 µg/ml for ciprofloxacin and amoxicillin.12,13
Statistical analysis
The Epi Info 2000 software package (Centers for Disease Control and Prevention, Atlanta, USA) was used for statistical analysis. Comparisons of antimicrobial resistance rates with the sex of the patients were determined using the chi-square test and a p-value of <0.05 was considered significant.
Results
Antimicrobial patterns
Of the 200 strains subjected to the antibiotics, 100% susceptibility was recorded for ciprofloxacin and 97.5% for amoxicillin. Marked resistance was noted for metronidazole 95.5% (Table I).
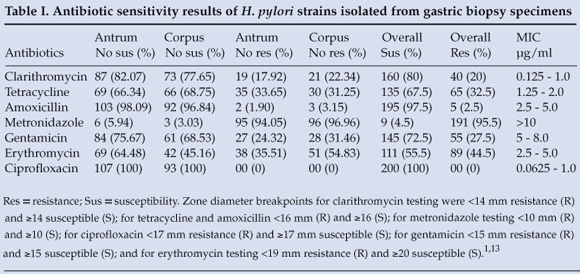
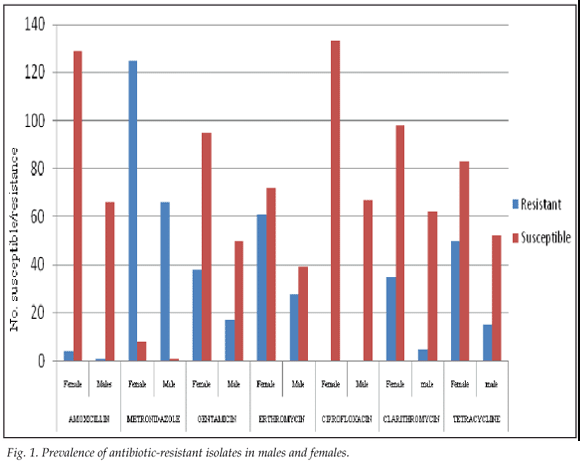
Of the 200 strains tested, 67 (33.5%) were from males and 133 (66.5%) from females. The prevalence of metronidazole resistance in females and males was 125/191 (65.44%) and 66/191 (34.55%) respectively, while for erythromycin it was 61/89 (68.53%) and 28/89 (31.46%) respectively (Fig. 1). There was a higher prevalence of resistant isolates in female compared with male patients, that did not reach statistical significance (p>0.05).
MIC determination
Of the 7 antibiotics, metronidazole showed no MIC within the susceptible breakpoint range. MIC values for the other antibiotics were 0.0625 - 1.0 µg/ml for ciprofloxacin, 0.125 - 1.0 µg/ml for clarithromycin, 1.25 - 2.0 µg/ml for tetracycline, and 2.5 - 5 µg/ml for amoxicillin (Table I).
Discussion
The resistance of H. pylori to antibiotic treatment regimens is a growing problem,14 and in several African countries is largely linked to misuse of antibiotics.1 Our results revealed marked antimicrobial susceptibility rates of 100% for ciprofloxacin, and 97.5% for amoxicillin, in line with other investigators.6,15 Hung et al.6 documented 94.3% susceptibility of their strains to ciprofloxacin, and Kohanteb et al.15 reported 95.3% for theirs. The latter authors also reported very high rates of amoxicillin susceptibility ranging from 79 - 99%. Although the rate of amoxicillin resistance is low in this study, and was absent for ciprofloxacin, resistance for both drugs has been demonstrated in different parts of the world.16
A similar study in western Nigeria documented 100% resistance by H. pylori strains to amoxicillin.7 We theorise that this could be related to the differences in local antibiotic prescription practices and use.2 H. pylori has been reported to lose the resistant phenotype to amoxicillin because of freezing and storage,14 which most probably occurred in the different studies; this may also help to explain the observed differences.1
Amoxicillin resistance is thought to develop because of structural alterations in the penicillin-binding protein, or changes in proteins involved in cell wall synthesis.17 The possibility of bacterial strains acquiring resistance to amoxicillin is therefore strong. Colonisation of the stomach with β-lactam-resistant bacteria may lead to the transfer of amoxicillin-resistance to H. pylori.
Our isolates were 80% susceptible to clarithromycin, 72.5% to gentamicin, 67.5% to tetracycline and 55.5% to erythromycin. Similar results were found in Ethiopia, Cameroon and Kenya, reporting very good activity of these antibiotics against H. pylori isolates.1 Others, however, have reported resistance to clarithromycin.14 Although clarithromycin is an expensive macrolide, cross-resistance linked with the use of other less expensive macrolides may be responsible for this resistance, since it is less abused.14 However, clarithromycin-susceptible and resistant strains have been isolated from patients with no history of exposure to macrolides,18 which may suggest that administration of clarithromycin may select for the resistant strains and therefore must be guided by empirical treatment.
We observed very high resistance (95.5%) to metronidazole, similarly to a study in Nigeria (100% resistance).7 Metronidazole resistance has been reported in 10 - 50% of all adult patients infected with H. pylori in developed countries,19 and virtually all strains are resistant in the developing countries.14 The high prevalence of metronidazole resistance in developing countries has been linked to the frequent use of nitroimidazole derivatives for the treatment of protozoal infections and gynaecological problems.1,2,14 This resistance is thought to be mostly because of genetic mutations in the RdxA and FrxA genes.5
We found no significant difference (p<0.05) in the prevalence of antibiotic-resistant strains among females and males, although there was a remarkable difference in metronidazole resistance between the sexes, as seen in other studies.14 However, the higher resistance observed with metronidazole in females may be related to its use in treating trichomoniasis and bacterial vaginosis, which is common in most developing countries.1,14
In our study, MIC values for the antibiotics that demonstrated susceptibility to the isolates were 0.125 - 1.0 µg/ml for clarithromycin, 1.25 - 2.0 µg/ml for tetracycline, 2.5 - 5.0 µg/ml for amoxicillin,; 5 - 8.0 µg/ml for gentamicin, 2.5 - 5.0 µg/ml for erythromycin, and 0.625 - 1.0 µg/ml for ciprofloxacin. These values are similar to those found in other studies,14 and are in agreement with CLSI-approved quality control ranges for H. pylori.12,13
Conclusion
We commonly encountered multidrug resistance of clinical significance that calls for continuous surveillance of antibiograms to guide empirical treatment. We advocate the inclusion of ciprofloxacin in the treatment regimen of H. pylori infection in our study environment. Our results also indicate the need to establish baseline susceptibility data for empirical treatment of cases and the conducting of studies involving newer and broad-spectrum antibiotics to address resistance.
We are grateful to the National Research Foundation of South Africa (grant reference 69815) and Govan Mbeki Research and Development Centre, University of Fort Hare, Alice, for financial support, and the staff of the GIT Unit, Livingstone Hospital, Port Elizabeth, and Mr B Clarke, for technical assistance.
References
1. Ndip RN, Takang AEM, Ojongokpoko JEA, et al. Helicobacter pylori isolates recovered from gastric biopsies of patients with gastro-duodenal pathologies in Cameroon: Current status of antibiogram. Trop Med Int Health 2008; 13(6): 848-854. [ Links ]
2. Tanih NF, Dube C, Green E, et al. An African perspective on Helicobacter pylori prevalence, drug resistance and alternative approaches to treatment. Ann Trop Med Parasitol 2009; 103(3): 189-204. [ Links ]
3. Pounder RE, Ng D. The prevalence of Helicobacter pylori infection in different countries. Aliment Pharmacol Ther 1995; 9 (suppl 2): 33-39. [ Links ]
4. NIH consensus Development Panel on Helicobacter pylori in peptic ulcer disease. Helicobacter in peptic ulcer disease. JAMA 1994; 272: 65-69. [ Links ]
5. Kwon, DH, Hulten, K, Kato, M, et al. DNA sequence analysis of rdxA and frxA from 12 pairs of metronidazole-sensitive and -resistant clinical Helicobacter pylori isolates. Antimicrob Agents Chemother 2001; 45(9), 2609-2615. [ Links ]
6. Hung KH, Sheu BS, Chang WL, Wu HM, Liu CC, Wu JJ. Prevalence of primary fluoroquinolone resistance among clinical isolates of Helicobacter pylori at a university hospital in Southern Taiwan. Helicobacter 2009; 14(1): 61-65. [ Links ]
7. Smith SI, Oyedeji KS, Arigbabu AO, Atimomo C, Coker AO. High amoxicillin resistance in Helicobacter pylori isolated from gastritis and peptic ulcer patients in Western Nigeria. J Gastroenterol 2001; 36: 67-68. [ Links ]
8. Kim JJ, Reddy R, Lee M, et al. Analysis of metronidazole, clarithromycin and tetracycline resistance of Helicobacter pylori isolates from Korea. J Antimicrob Chemother 2001; 47: 459-461. [ Links ]
9. Aboderin OA, Abdu AR, Odetoyin B, et al. Antibiotic resistance of Helicobacter pylori from patients in Ile-Ife, south-west, Nigeria. Afr Health Sci 2007; 7(3): 143-147. [ Links ]
10. Louw JA, Marks IN. The treatment of peptic ulcer disease. Curr Opin Gastroenterol 1999; 15(6): 497-503. [ Links ]
11. Wong BC, Chang FY, Abid S, et al. Triple therapy with clarithromycin, omeprazole, and amoxicillin for eradication of Helicobacter pylori in duodenal ulcer patients in Asia and Africa. Aliment Pharmacol Ther 2000; 14(11): 1529-1535. [ Links ]
12. Performance standards for antimicrobial susceptibility testing; fifteenth information supplement. Wayne, PA, USA: Clinical and Laboratory Standards Institute, 2007. [ Links ]
13. Osato MS. Antimicrobial susceptibility testing for Helicobacter pylori: sensitivity test results and their clinical relevance. Curr Pharm Des 2000; 6: 1545-1555. [ Links ]
14. Nahar S, Mukhopadhyay AK, Khan R, et al. Antimicrobial susceptibility of H. pylori strains isolated in Bangladesh. J Clin Microbiol 2004; 42: 4856-4858. [ Links ]
15. Kohanteb J, Bazargani A, Saberi-Firoozi M, Mobasser A. Antimicrobial susceptibility testing of Helicobacter pylori to selected agents by agar dilution method in Shiraz-Iran. Indian J Med Microb 2007; 25: 374-377. [ Links ]
16. Quintana-Guzmán, EM, Arias-Echandi, ML, Salas-Chaves P, Davidovich-Rose H, Schosinsky-Neverman K. Helicobacter pylori: susceptibility to amoxycillin, erythromycin, tetracycline, ciprofloxacine, nitrofurantoin and metronidazole in Costa Rica. Rev Biomed 1998; 9: 92-96. [ Links ]
17. Deloney CR, Schiller NL. Characterization of an in vitro selected amoxicillin-resistant strain of H. pylori. Antimicrob Agents Chemother 2000; 44: 3363-3373. [ Links ]
18. Matsuoka M, Yoshida Y, Hayakawa K, Fukuchi S, Sugano K. Simultaneous colonization of Helicobacter pylori with and without mutations in the 23SrRNA gene in patients with no history of clarithromycin exposure. Gut 1999; 45: 503-507. [ Links ]
19. Adamek RJ, Suerbaum S, Pfatenbach B, Operkuch S. Primary and acquired Helicobacter pylori resistance to clarithromycin, metronidazole and amoxicillin influence on treatment outcome. Am J Gastroenterol 1998; 93: 386-389. [ Links ]
Accepted 19 June 2009.
* Corresponding author: R Ndip (rndip@ufh.ac.za)














