Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
SAMJ: South African Medical Journal
versión On-line ISSN 2078-5135
versión impresa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.100 no.1 Pretoria ene. 2010
ORIGINAL ARTICLES
Utilisation and outcomes of cervical cancer prevention services among HIV-infected women in Cape Town
Priya BatraI,*; Louise KuhnII; Lynette DennyIII
IBA, College of Physicians and Surgeons, Columbia University, New York
IIPhD, Mailman School of Public Health, Columbia University
IIIMB ChB, MMed, PhD, FCOG (SA), Department of Obstetrics and Gynaecology, University of Cape Town and Groote Schuur Hospital, Cape Town
ABSTRACT
OBJECTIVE: An audit of outcomes of cervical cancer screening and prevention services for HIV-positive women in Cape Town, South Africa.
DESIGN: Retrospective review of clinic registers, patient records and pathology databases at three HIV primary health clinics and a tertiary colposcopy referral centre.
SUBJECTS: Women recently diagnosed with HIV at three primary health clinics between 2006 and 2008 (N=2 240); new patients seen for colposcopy at a tertiary referral centre between 2006 and 2009 (N=2 031).
OUTCOME MEASURES: The proportion of women undergoing cervical cancer screening after HIV diagnosis at primary health clinics, demographic characteristics of women referred for colposcopy at a tertiary centre, and outcomes of therapy for precancerous lesions of the cervix.
RESULTS: The proportion of women undergoing at least one Pap smear at HIV primary health clinics after HIV diagnosis was low (13.1%). Women referred for colposcopy tended to be HIV-positive and over the age of 30 years, and in most (70.2%) cytological examination revealed high-grade cervical dysplasia. HIV-positive women treated with excision for precancerous lesions of the cervix were significantly more likely than their HIV-negative counterparts to undergo incomplete excision, experience persistent cervical disease after treatment, and be lost to follow-up.
CONCLUSION: Cervical cancer screening efforts must be scaled up for women with HIV. Treatment and surveillance guidelines for cervical intraepithelial neoplasia in HIV-positive women may need to be revised and new interventions developed to reduce incomplete treatment and patient default.
Cervical cancer is the most common cancer among South African women. The age-standardised mortality rate from cervical cancer is 21.0% in South Africa but only 8.9% worldwide.1,2 This differential highlights inequality in the availability of screening for easily treated precursor lesions. South Africa's cervical cancer burden severity is compounded by its HIV epidemic (2008 adult prevalence rate 18.1%).3 In HIV-infected women, the risk of developing squamous intraepithelial lesions (SIL) is increased, and the annual incidence of human papillomavirus (HPV)-associated cervical intraepithelial neoplasia (CIN) is 4 - 5 times greater than in the general population.4,5 CIN appears in younger women and progresses to cancer more quickly in the presence of HIV.6
Cancer screening rates in South Africa are low, regardless of HIV status. Papanicolaou (Pap) screening rates are as low as 4% among women 15 - 65 years of age, with most performed in antenatal or family planning clinics.7 In 2000, the South African Department of Health published guidelines for a comprehensive national cervical cancer screening programme. All asymptomatic women of at least 30 years of age should be offered three free lifetime Pap smears, up to 10 years apart.8 This policy did not take HIV status into account, and there are few evidence-based guidelines for cervical cancer screening in HIV-positive women.
We aimed to evaluate the provision of cervical cancer prevention services to HIV-positive women in Cape Town. First, we attempted to define the proportion of HIV-positive women undergoing Pap smears at HIV primary health clinics (PHCs) in the community. Second, we audited the Groote Schuur Hospital (GSH) colposcopy clinic to describe the impact of HIV status on diagnosis and management of precursors to cervical cancer.
Methods
Study design and subjects
We conducted a retrospective review of clinic registers, patient medical records and pathology databases at three HIV PHCs in Cape Town and the GSH colposcopy clinic, which is a tertiary referral site for a large proportion of women undergoing cervical screening in Cape Town.
PHCs serving HIV-positive women
The PHC sample included all women at three selected HIV clinics who were aged at least 16 years, had been tested for HIV between 1 August 2006 and 31 July 2008, and were receiving care for HIV. Western Cape Department of Health policy specifies that HIV-positive women should undergo annual cervical screening by Pap smear, the first performed just after HIV diagnosis. The three HIV PHC sites selected were known to refer patients with abnormal smears to GSH for colposcopy. Data captured included PHC location, age, whether the woman underwent a Pap smear, cytology results and any details regarding referral for colposcopy. For the subject to be included, Pap smears had to have been performed after HIV diagnosis and before 1 December 2008.
GSH colposcopy clinic
This sample included all women at least 16 years of age referred between 1 January 2006 and 31 December 2008 as new patients for colposcopy from family planning clinics, community health centres and workplace clinics in Cape Town. Indications for referral to colposcopy were an abnormal glandular lesion on Pap smear, a single result of high-grade SIL or cancer, or two or more low-grade SIL or atypical squamous results on Pap smears taken at least 6 months apart. Patient encounters occurring before 1 January 2009 were reviewed.
The final sample included 2 045 women, of whom 2 031 had records available for review (99.3% completion). Data were collected for the first colposcopy visit for all subjects. For subjects undergoing large loop excision of the transformation zone (LLETZ) for treatment of CIN, data were also collected for the treatment visit and for the 4-month post-procedure visit. Indicators captured from colposcopy charts and pathology databases included demographics (age, gravidity/parity, co-morbid genital warts/herpes, co-morbid pulmonary tuberculosis, contraceptive use), self-reported HIV status, self-reported use of highly active antiretroviral therapy (HAART), smoking status, Pap smear results, colposcopy findings, histological diagnoses and compliance with follow-up visits. Laboratory results (cytology, histology) were recorded as reported by the National Health Laboratory Service (NHLS), which serves all PHC sites and GSH.
Statistical analysis
Data were entered into an anonymous database using Access 2000 software (Microsoft, Seattle, WA), and exported into SAS 9.1.3 (SAS Institute, Cary, NC) for analysis. Proportions of subjects undergoing Pap tests were calculated for PHC sites. Descriptive statistics were generated for demographic data in the GSH sample. We used the chi-square (χ2) statistic to test for differences in outcomes by categorical variables, with statistical significance defined at alpha = 0.05 (p=0.050). Outcome variables included whether a Pap smear was performed, colposcopy findings and cytology/histology pre- and post-treatment. Hypothesised determinant variables included age, contraceptive method, self-reported HIV status and HAART use.
Ethics considerations
Ethics approval was obtained from the Research Ethics Committee of the University of Cape Town Health Sciences Faculty and from the Columbia University Institutional Review Board (New York, USA).
Results
Pap smears performed among HIV-positive women at PHCs
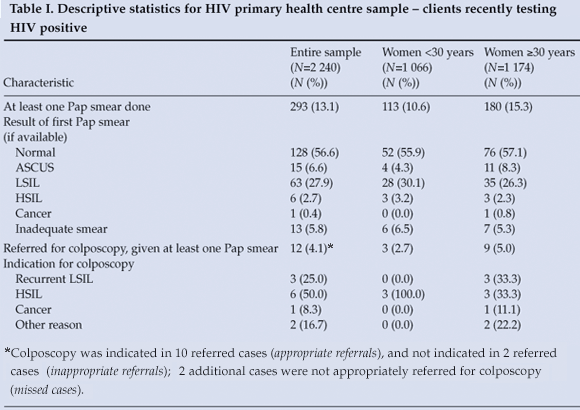
The final PHC sample included 2 240 HIV-positive women at three clinic sites (Table I); 52.4% were 30 years of age or older, and only 293 (13.1%) had had at least one Pap smear between HIV diagnosis and the end of the data collection period. Older women were significantly more likely to have had at least one Pap smear (p=0.0021). The proportion of women having at least one Pap smear was stratified by clinic site (A, B or C). At site A, 688 women tested HIV positive, of whom 72 (10.5%) had a Pap smear, compared with 92 of 1 346 patients (6.8%) at site B. At Site C, with a sample of only 206 HIV-positive women, 129 (62.6%) had Pap smears. Table I lists the PHC Pap smear results. Cytology results are reported as classified by the NHLS: normal, atypical squamous cells of undetermined significance (ASCUS), atypical squamous cells – cannot exclude high-grade SIL (ASC-H), low-grade SIL (LSIL), high-grade SIL (HSIL), atypical glandular cells of undetermined significance (AGUS), adenocarcinoma in situ, or cancer. Most women were found to have normal, ASCUS or LSIL Pap smears. Referrals for colposcopy at GSH were indicated for 12 (4.1%) of the patients undergoing at least one Pap test, only 5 of whom had colposcopy (58.3% lost to follow-up after Pap smear).
GSH colposcopy clinic data – descriptive analysis
The final GSH sample included 2 031 women (Table II); 70.0% were between 30 and 49 years of age. Over half of all new patients were HIV positive by self-report. No contraception was used by 633 (31.2%), while just over one-third were using long-acting injectable progestins (LAIP). Condom use was reported by 210 (10.3%), with only 175 (17.1%) of HIV-positive patients reporting regular condom use. HAART was utilised by 524 (51.3%) of HIV-positive women at the time of their index colposcopy visits (self-reported). Co-morbid pulmonary tuberculosis, genital herpes and genital warts were significantly more likely to be reported in HIV-positive patients (p<0.0001, p=0.0026 and p<0.0001, respectively).
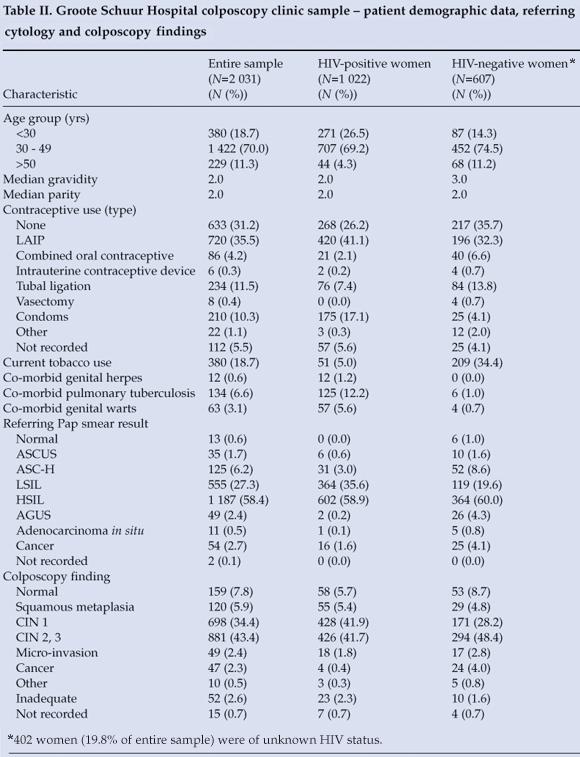
Table II presents referring Pap smear and colposcopy findings. An HSIL Pap smear was the reason for referral in 1 187 (58.4%) women. Consistent with referring Pap smear results, most women were considered to have CIN 2 or 3 (high-grade dysplasia) by the colposcopist. LSIL Pap smears were seen in 555 (27.3%) of women referred to GSH; 364 (65.6%) of them were HIV positive by self-report, and 335 (60.4%) were also found to have low-grade dysplasia on colposcopy.
LLETZ outcomes
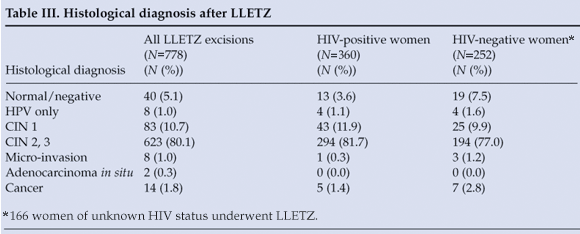
LLETZ was performed in 778 patients for treatment of CIN (Table III), of whom 360 (46.3%) were HIV positive and 186 (51.7%) reported using HAART. The loss to follow-up rate after LLETZ (patient did not attend 4-month post-procedure visit) was 21.9% for patients seen in 2006 and 2007 (LLETZ cases from 2008 were excluded, as patients may have been scheduled for follow-up after the close of data collection). HIV-positive women were significantly more likely than HIV-negative women to be lost to follow-up after LLETZ (p=0.0280). Descriptive statistics were calculated for the presence of residual CIN at LLETZ ecto- and endocervical excision margins. Both margins were negative in 349 (44.9%) of LLETZ cases, and one or both margins were positive for residual CIN in 429 cases (55.1%). HIV-positive women undergoing LLETZ were significantly more likely to have one or more margins positive for residual CIN (p<0.0001).
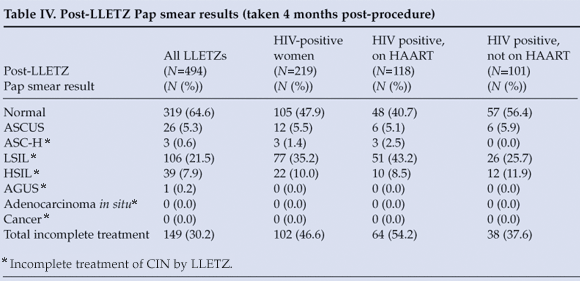
Post-LLETZ Pap smear results were available for 494 women (Table IV). LSIL or a more severe lesion on Pap smear (defined as 'incomplete treatment') was seen in 149 women (30.2%) 4 months after LLETZ. HIV-positive women were significantly more likely than HIV-negative women to be incompletely treated by LLETZ (p<0.0001). The 'incomplete treatment' group was also significantly more likely to have both excision margins positive for residual CIN (p<0.0001). Use of LAIP contraceptives, histological grade and self-reported HAART use (in HIV-positive subjects) were not significantly associated with LLETZ treatment success.
Positive predictive value of colposcopy
Positive predictive value (PPV) describes the proportion of patients with a positive diagnostic test result subsequently found to have true disease on confirmatory testing. We examined the subgroup of women classified as having CIN 1 or more severe lesion on colposcopy and identified the proportion in this subgroup who had CIN 1 or more severe lesion confirmed by histological sampling (biopsy or LLETZ) to calculate PPV. The PPV of colposcopy in predicting a histological diagnosis of CIN 1 or more severe lesion was 82.6% among women who had diagnostic tissue sampling and for whom adequate colposcopy results were available (1 787 of the sample of 2 031 women). PPV remained robust when stratified by self-reported HIV status. In HIV-positive women with colposcopy findings and histological results available (N=897) the PPV was 83.0%, and in HIV-negative women (N=537) it was 81.1%.
Discussion
Primary health centre data on recently diagnosed HIV-positive women revealed that the proportion of patients undergoing at least one Pap smear was low (13.1%). HIV-positive women over the age of 30 years were preferentially screened at HIV clinics. It is possible (though unlikely) that HIV-positive women undergo cervical screening at other primary health care sites. PHC site C, which served the fewest patients and offered primary care and HIV services in a single setting, provided almost two-thirds of newly diagnosed HIV-positive women with at least one Pap test. Larger HIV clinic sites – with greater patient volumes and more fragmented service provision – were less successful in linking cervical cancer prevention to HIV care.
Over 80% of women referred to GSH for colposcopy were at least 30 years old. It appears that primary health care providers are systematically offering Pap smears in accordance with national cervical screening guidelines, and are shifting away from opportunistic screening provided in the context of antenatal or family planning care. HIV-positive women were over-represented in the colposcopy sample. When combined with our data from primary care clinics, this finding suggests that while Pap rates among HIV-positive women may be low, the proportion of HIV-negative women undergoing Pap smears may be even lower. HIV-positive women in Cape Town may have more frequent contacts with the health care system. Many women referred for colposcopy also utilised contraceptive methods requiring regular health care encounters (i.e. LAIP, tubal ligation). Cape Town women with regular health system interactions of any type may have more lifetime Pap smear opportunities.
Colposcopy had a PPV of 82.6%, which was not surprising given that PPV is directly proportional to disease prevalence (known to be high in our sample). It is important to note that PPV was not altered by self-reported HIV status. Colposcopy has previously been demonstrated to correlate as well with histological diagnosis in HIV-positive women as in uninfected controls.9 While colposcopy is useful in estimating lesion grade, management decisions may still require histological confirmation.
A substantial proportion of women referred for colposcopy (27.3%) had low-grade SIL according to cytology, and most of these were HIV positive. Although the relative risk of progression of low-grade lesions to high-grade CIN/cancer is increased in HIV-positive women, the absolute risk of progression remains low.10 In a study of HIV-positive women from Cape Town, Denny et al. demonstrated that while 35% of women had LSIL on Pap smear at entry, only 4% of them progressed to high-grade SIL over a 36-month follow-up period.4 However, referrals of low-grade dysplasia to GSH probably represent a small fraction of the true number of women requiring colposcopy, given the low Pap smear rates reported at PHC sites, and the requirement of two low-grade Pap smear abnormalities for colposcopy referral. As cervical screening increases, the number of cases of low-grade cervical disease requiring follow-up is likely to outpace growth in colposcopy services.
We found a post-LLETZ patient default rate of over 20%. This is concerning, as HIV-positive women were most often lost to follow-up. Those HIV-positive women who did follow up were significantly more likely to present with Pap smear abnormalities after LLETZ. Our suboptimal LLETZ treatment outcomes mirror studies in South Africa, Brazil and the USA.11-13 HIV-positive women may require more intensive surveillance for persistent cervical abnormalities after LLETZ. HAART therapy was not associated with improved excision outcomes in HIV-positive women in our sample. Studies on HAART and CIN excision are mixed; while some suggest that HAART promotes regression of CIN/SIL after excision,14 others have not shown any clear cervix-specific treatment benefit.15 However, HAART use was self-reported in our sample, and we had no data on duration of therapy.
Limitations and further directions
A retrospective design limited this study's findings, as research questions were restricted to data recorded in clinic folders and registers. Data on severity of HIV-related disease (i.e. CD4 count, viral load) were unavailable, and the impact of these factors on study outcomes could not be assessed. HIV status was self-reported in the GSH sample – many women who reported 'negative' HIV status had last been tested more than 12 months before index colposcopy encounters. The large proportion of HIV-positive women in our sample may be an underestimate.
Our study illustrates the realities of Cape Town's cervical cancer prevention programme for women living with HIV. The proportion of HIV-positive women undergoing Pap smears at HIV care sites is low, and varies highly by clinic site. In spite of low Pap test rates, many HIV-positive women are being referred for colposcopy. Poor follow-up after excision coupled with suboptimal CIN treatment outcomes in HIV-positive women means that cervical surveillance efforts must be scaled up for these patients. New recommendations for follow-up in these women will impact on the allocation of health resources for cervical cancer prevention. Cervical cancer prevention must be linked to basic HIV care for health outcomes to improve.
Dr R Soeters assisted in the design of data collection instruments, and ensured institutional support. Drs N Mbatani, P van Greunen, R Boa, B Howard, K Dehaeck and J Robinson of Groote Schuur Hospital provided assistance. Dr M Sobieszczyk of Columbia University provided guidance during study development. The investigators thank the Western Cape Department of Health for its support.
Conflict of interest. The authors have nothing to disclose.
Funding support. The Doris Duke Charitable Foundation.
References
1. World Health Organization/Institut Catala d'Oncologia. Human Papillomavirus and Cervical Cancer Summary Report: South Africa [document on the Internet]. Barcelona: WHO/ICO, 2007 (updated 2007). http://www.who.int/hpvcentre/en/ (accessed 10 May 2009). [ Links ]
2. Denny L. Prevention of cervical cancer. Reprod Health Matters 2008; 16: 18-31. [ Links ]
3. Joint United Nations Programme on HIV/AIDS and the World Health Organization Working Group on Global HIV/AIDS and STI Surveillance. Epidemiological Fact Sheet on HIV and AIDS: South Africa [document on the Internet]. Geneva: UNAIDS/WHO, 2008 (updated September 2008). http://data.unaids.org/ Publications/Fact-Sheets01/southafrica_EN.pdf (accessed 10 May 2009). [ Links ]
4. Denny L, Boa R, Williamson AL, et al. Human papillomavirus infection and cervical disease in human immunodeficiency virus-1-infected women. Obstet Gynecol 2008; 111: 1380-1387. [ Links ]
5. Ellerbrock TV, Chiasson MA, Bush TJ, et al. Incidence of cervical squamous intraepithelial lesions in HIV-infected women. JAMA 2000; 283: 1031-1037. [ Links ]
6. Danso D, Lyons F, Bradbeer C. Cervical screening and management of cervical intraepithelial neoplasia in HIV-positive women. Int J STD AIDS 2006; 17: 579-584. [ Links ]
7. Cronje HS, Beyer E. Screening for cervical cancer in an African setting. Int J Gynaecol Obstet 2007; 98: 168-171. [ Links ]
8. South African Department of Health. National Guideline On: Cervical Cancer Screening Programme [document on the Internet]. Pretoria: SA DOH, 2000 (updated 2000). http://www.doh.gov.za/docs/index.html (accessed 10 May 2009). [ Links ]
9. Massad LS, Schneider M, Watts H, et al. Correlating Papanicolaou smear, colposcopic impression, and biopsy: Results from the Women's Interagency HIV Study. J Low Genit Tract Dis 2001; 5: 212-218. [ Links ]
10. Massad LS, Evans CT, Minkhoff H, et al. Natural history of grade I cervical intraepithelial neoplasia in women with human immunodeficiency virus. Obstet Gynecol 2004; 104: 1077-1085. [ Links ]
11. Adam Y, van Gelderen CJ, de Bruyn G, McIntyre JA, Turton DA, Martinson NA. Predictors of persistent cytologic abnormalities after treatment of cervical intraepithelial neoplasia in Soweto, South Africa: a cohort study in a HIV high prevalence population. BMC Cancer 2008; 8: 211-221. [ Links ]
12. Lima MI, Tafuri A, Araujo AC, de Miranda Lima L, Melo VH. Cervical intraepithelial neoplasia recurrence after conization in HIV-positive and HIV-negative women. Int J Gynaecol Obstet 2009; 104: 100-104. [ Links ]
13. Massad LS, Fazzari MJ, Anastos K, et al. Outcomes after treatment of cervical intraepithelial neoplasia among women with HIV. J Low Genit Tract Dis 2007; 11: 90-97. [ Links ]
14. Ahdieh-Grant L, Li R, Levine AM, et al. Highly active antiretroviral therapy and cervical squamous intraepithelial lesions in human immunodeficiency virus-positive women. J Natl Cancer Inst 2004; 96: 1070-1076. [ Links ]
15. Soncini E, Zoncada A, Condemi V, Antoni AD, Bocchialini E, Soregotti P. Reduction of the risk of cervical intraepithelial neoplasia in HIV-infected women treated with highly active antiretroviral therapy. Acta Biomed 2007; 78: 36-40. [ Links ]
Accepted 24 August 2009.
* Corresponding author: P Batra (priya.batra@gmail.com)














