Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.99 n.12 Pretoria Dec. 2009
ORIGINAL ARTICLES
Solubility tests and the peripheral blood film method for screening for sickle-cell disease: a cost benefit analysis
Andrew Livex OkwiI, *; Michael OcaidoIV; Wilson ByarugabaII; Christopher Magala NdugwaIII; Arthur ParkesV
IDMLT, MSc. Department of Pathology, Medical School, Makerere University, Kampala, Uganda
IIMSc, PhD. Department of Pathology, Medical School, Makerere University, Kampala, Uganda
IIIMB ChB, MMed, DTM&H. Department of Paediatrics and Child Health, Medical School, Makerere University, Kampala, Uganda
IVPhD. Department of Wildlife and Animal Resources, Faculty of Veterinary Medicine, Makerere University, Kampala, Uganda
VMSc, PhD. Centre of Endocrine and Diabetes Science, Cardiff University, Cardiff, UK
ABSTRACT
OBJECTIVE: To determine the cost benefit of screening for sickle-cell disease among infants at district health centres in Uganda using sickling, solubility tests and the peripheral blood film method.
METHODS: Pilot screening services were established at district health centres. Cost benefit analysis (CBA) was performed in four scenarios: A1 – where there are no sickle-cell screening services at district health centres and all children are referred either to Mulago national referral hospital or A2 – a regional hospital for haemoglobin (Hb) electrophoresis; B1 – when there are screening services at district health centres, only positive samples are taken either to Mulago Hospital or B2 – the regional hospital for confirmation using haemoglobin electrophoresis. Calculations were done in Uganda shillings (USh).
RESULTS: Initial operational costs were high for all scenarios but variably reduced in the subsequent years. Scenarios A1 and A2 were very sensitive compared with B1 and B2. Scenario A1 had the highest screening costs in the subsequent years, costing over 62 000 USh per test in both eastern and western Uganda. Scenario B2 was sensitive and cheaper when using the sickling test, but was expensive and insensitive when using the solubility test and more insensitive though cheaper when using the peripheral blood film method.
CONCLUSION AND RECOMMENDATION: Screening children in Mulago hospital using haemoglobin electrophoresis (A1) was very expensive although it was sensitive. Screening the children at four health centres using the sickling method and confirming positive samples at a regional hospital (B2) was both cheap and sensitive and is therefore recommended.
Sickle-cell disease (SCD) is one of the haemoglobinopathies responsible for high morbidity and mortality among neonates in developing countries, including Uganda.1 It is therefore necessary to establish the most cost-effective screening and intervention programmes to reduce morbidity and mortality among children with SCD.2 However, most governments in developing countries, including Uganda, cannot fund all the necessary medical programmes because of scarce resources. Cost benefit analyses are therefore performed to determine which programmes or options offer the greatest benefits at the lowest cost.3,4
While some developing countries such as Jamaica and Ghana have made progress in introducing comprehensive sickle-cell screening programmes,5,6 the peripheral blood film method for screening for SCD at district health centres has been recommended in Kenya.7 In Malawi, the most cost-effective haemoglobinopathy testing methods have been recommended to be used at district hospitals.8
Since no cost benefit analysis (CBA) studies have been done on sickle-cell screening methods in Uganda, we studied the cost benefit of screening for SCD among infants at district health centres using the sickling and solubility tests and the peripheral blood film method.
Methods and materials
Pilot screening services were established using sickling and solubility tests and the peripheral blood film method at each selected health centre in each district where medical laboratory technicians had been trained. The trained technicians screened for SCD using these methods and then delivered blood samples to the Faculty of Medicine at Mulago Hospital, to be analysed using cellulose acetate membrane haemoglobin electrophoresis (gold standard).9 Each batch of assays included a negative control (haemoglobin AA) and positive control (haemoglobin SS).
CBA performed under several assumptions and different scenarios determined the benefits and/or profitability of each method. Cumulative costs in USh and benefits were compared over a 5-year period.
Analysis was based on the following assumptions:
Each of the sickle-cell screening methods established at the district health centre would be the recommended screening method for SCD.
The automated capillary haemoglobin electrophoresis system would be used in Mulago and regional hospitals as a 'gold standard'.
All children testing negative at district health centres are considered free of the disease except those who later develop symptoms.
Only children who test positive at the district health centres will have confirmatory testing, and those confirmed positive would benefit from interventions and survive.
Screening costs only, and not treatment costs, will be considered.
Persons performing these tests would also be doing routine work and would be paid a monthly salary.
Glassware not falling under consumables would be replaced in the second year.
The costs of running sickle-cell tests took into account the regional prevalence of SCD among newborn children.
The haemoglobin electrophoresis machine would depreciate at USh 11 571 750 per year, while each microscope and weighing balance would depreciate at USh 300 000 and USh 50 280 per year, respectively.
95% of children with the sickle cell trait (AS) would live a normal life, and 85% with sickle cell anemia (SS) would live to adulthood if diagnosed and appropriately treated.10
The following scenarios were used:
A1: there are no sickle-cell screening services at district health centres and regional hospitals, and all children are referred to Mulago Hospital for haemoglobin electrophoresis
A2: there are no screening services at district health centres and all children are referred to the regional hospital for haemoglobin electrophoresis
B1: there are sickle-cell screening services at district health centres and only positive samples are brought to Mulago Hospital
B2: there are screening services at district health centres and only positive samples are brought to the regional hospital for confirmation.
Results
Technicians took an average of 38 minutes to prepare the sickling test solution and perform the test, and an average of 70 minutes to prepare working solutions and perform the solubility test. Preparing peripheral blood film method solutions and performing the test took an average of 44 minutes. Technicians took 6 minutes on average to bleed each child.
A total of 286 children were screened in eastern Uganda (March - May 2007) and 370 in western Uganda (June - August 2007). In eastern Uganda, haemoglobin electrophoresis detected 50 AS and 5 SS cases , while the sickling test demonstrated the presence of 26 AS, 5 SS, the solubility test 13 AS, 4 SS and the peripheral blood film method 7 AS and 2 SS. In the west, haemoglobin electrophoresis detected 11 AS and no SS, while the sickling test demonstrated the presence of 5 AS, the solubility test 3 AS and the peripheral smear 2 AS. Reliability was performed separately using haemoglobin electrophoresis as a gold standard, and a sample size of 200 blood samples was used. The sickling test had the highest sensitivity and specificity of 65% and 95% respectively compared with solubility, which had 45% and 90%, and peripheral blood film 35% and 96.7%, respectively.11
In scenario A1, the cost of USh 75 665 253 would be incurred in the first 3 months in eastern Uganda and USh 61 822 853 would be incurred if scenario A2 intervention activities were adopted, thereby saving USh 13 842 400. In western Uganda, USh 82 005 135 would be incurred in scenario A1 and USh 63 727 135 would in scenario A2, thereby saving USh 18 278 000 (Table I).

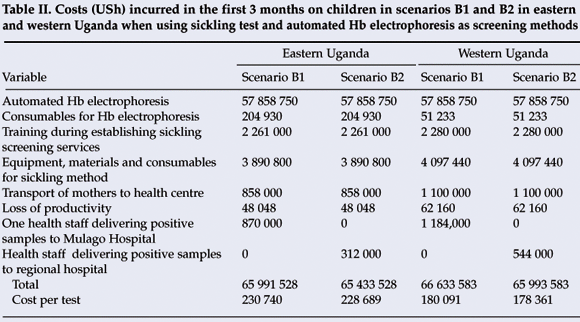
It would cost USh 65 991 528 using the sickling test in eastern Uganda in the first 3 months in scenario B1 and USh 65 433 528 in B2, thereby saving USh 558 000. In western Uganda USh 66 633 583 would be needed in the first 3 months in B1 and USh 65 993 583 in B2, thereby saving USh 640 000 (Table II).
Using the solubility test, USh 63 530 316 would be spent in eastern Uganda in the first 3 months under scenario B1 and USh 62 972 316 under B2 – a saving of USh 558 000. In western Uganda, USh 64 128 213 would be incurred in scenario B1 and USh 63 488 213 under B2, therefore saving USh 640 000 (Table III).

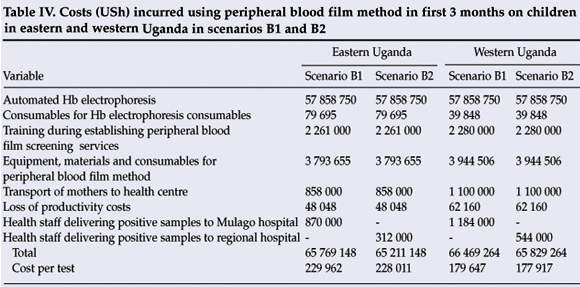
Using the peripheral blood film method, it would cost USh 65 769 148 in the east in the first 3 months using scenario B1, and USh 65 211 148 in B2, thereby saving USh 558 000. In the west, USh 66 469 264 would be incurred in scenario B1 in the first 3 months, and USh 65 829 264 in B2, thereby saving 640 000 (Table IV).
These screening programmes would all save the same amount of money in the first 3 months in both eastern and western Uganda.
The costs of the screening services were projected for a period of 5 years, and it was assumed that more children with SCD would be detected and more money would be saved because the only costs incurred would be service maintenance costs. These were calculated annually on the incremental basis of 3% of the previous year's maintenance costs, since there would be an expected 3% annual increase in the general population.12 Other costs included depreciation of laboratory equipment calculated at each closing fiscal year by dividing the total cost of the equipment by its useful life years, taken as 5 years for medical equipment.13
When the sickling test is used together with the haemoglobin electrophoresis method, USh 9 565 912 would be incurred in eastern Uganda under scenario B1 and USh 7 147 912 in scenario B2 in year 1, thereby saving USh 2 418 000. In western Uganda, USh 10 920 130 would be incurred in scenario B1 and USh 8 840 130 in scenario B2, thereby saving USh 2 080 000. Using the solubility method, it would cost USh 11 766 602 in year 1 under scenario B1 and USh 9 348 602 in B2 in eastern Uganda, therefore saving USh 2 418 000. In western Uganda, a cost of USh 13 376 820 and USh 11 296 820 would be incurred in scenario B1 and B2 respectively, thereby saving USh 2 080 000. When the peripheral blood film method is used, USh 8 876 392 would be incurred in year 1 under scenario B1 and USh 6 458 392 in B2 in eastern Uganda, saving USh 2 418 000. In western Uganda, a cost of USh 10 262 854 would be incurred under scenario B1 and USh 8 182 854 under scenario B2, thereby saving USh 2 080 000. More money would be saved in the subsequent years.
Discussion
Cost benefit analyses are useful to identify the cheapest interventions with highest benefits.3 This was done on the solubility and sickling tests and the peripheral blood film method to determine the most cost-effective method for screening for SCD among neonates in the districts of Uganda.
The cost-effectiveness of screening for SCD was sensitive to various variables. As expected, it was sensitive to the prevalence, cost and reliability of the screening programme. While over USh 75 million would be incurred in scenario A1 in eastern Uganda in the first 3 months and more than USh 63 million in scenario A2, 50 children with AS and 5 with SS would be detected in eastern Uganda by A1 and A2, respectively.
While more than USh 82 million would be incurred on A1 in western Uganda and 63 million in B2, only 11 children with AS would be identified by both scenarios in the first 3 months. These differences were probably due to the high prevalence of SCD in eastern Uganda and the high reliability of the haemoglobin electrophoresis method. These findings agree with studies which found that identifying sick persons in a population with low prevalence or risk of the disease was associated with very high costs.2,14 Therefore screening the population in western Uganda, which has a very low prevalence of AS and SS, using the haemoglobin electrophoresis method would not be cost effective because of the high cost of identifying these children. In eastern Uganda the same programme would cost-effectively identify many of these children.3
Whereas both the sickling test and peripheral blood film method were more expensive than the solubility test in the first 3 months because of the high costs of equipment, they subsequently became cheaper. Although scenarios B2 and B1 were not as sensitive as scenarios A1 and A2, scenario B2 demonstrated the same number of cases with SS as scenarios A1 and A2 at low cost when using the sickling test, implying that the sickling method could cheaply and reliably be used for early detection of children with haemoglobin SS. Although the peripheral blood method for sickle-cell screening at district health centres has been recommended,7 we found it insensitive but cheap.
Scenarios A1 and A2 would continue saving more money throughout the subsequent years, while in scenarios B1 and B2 the same amount of money would be saved in both eastern and western Uganda. While the reasons for this are difficult to explain, some costs were estimated and were therefore not accurate, and could have influenced the findings.
Conclusions and recommendations
Screening all children in Mulago hospital using haemoglobin electrophoresis (scenario A1) was sensitive but not cost beneficial. We recommend screening all the children at sub-district health centres by means of the sickling method and confirming only positive samples at a regional hospital (B2), as it was the most cost-effective intervention.
We thank Innovations at Makerere University Committee (I@Mak.com) for sponsorship, the district leaders in charge of health centres for their contribution, the health staff for their cooperation, and Mrs H Ndugosa, P Ayika and P Byanyima for their participation.
References
1. Sergeant GR, Ndugwa CM. Sickle cell disease in Uganda: A time for action. East Afr Med J 2003; 80: 383-387. [ Links ]
2. Tsevat J, Wong JB, Pauker SG, Steinberg MH. Neonatal screening for sickle cell disease: A cost-effectiveness analysis. J Pediatr 1999; 118: 546-554. [ Links ]
3. Teutsch SM, Murray FJ. Dissecting cost-effectiveness analysis for preventive interventions. A guide for decision making. Am J Managed Care 1999; 5: 301-305. [ Links ]
4. Louise BR. Cost-effectiveness analysis and screening tests for women. JAMWA 2000; 55: 207-209. [ Links ]
5. Hays RJ, Searjean GR. Testing for random occurrence of sickle cell disease in a study of 100 000 Jamaican newborns. J Trop Med Hyg 1990; 93: 127-132. [ Links ]
6. Akinyanju OO. Situation of sickle cell and genetics services in Nigeria. 11th International Congress of Human Genetics, 2006. www.ichg2006.com/abstract/1205.htm (Accessed 11 October 2006). [ Links ]
7. Aluoch JR. The presence of sickle cell in peripheral blood film. Specificity and sensitivity of diagnosis of homozygous sickle cell disease in Kenya. Trop Geogr Med 1995; 47: (2) 89-91. [ Links ]
8. Medina LA, Mundy C, Kandulu J, Chisuwo L, Bates I. Evaluation and costs of different haemoglobin methods for use in district hospitals in Malawi. J Clin Pathol 2005; 58: 56-60. [ Links ]
9. Barbara JW, Barbara JB. Investigations of hemoglobinopathies and thalassemias. In: Lewis SM, Brain BJ, Bates I, eds. Practical Hematology. Edinburgh: Churchill Livingstone, 2001: 1815-1827. [ Links ]
10. Linda L, Dezateux C, Anionwu EN. Neonatal screening for sickle cell disorder: what about the carrier state? BMJ 1996; 313: 407-410. [ Links ]
11. Okwi AL, Ndugwa CM, Byarugaba W, Parkes A, Ocaido M, TumwinE JK. The reliability of the sickling, solubility tests and peripheral blood film method for screening for sickle cell disease in Uganda. South African Journal of Child Health (in press). [ Links ]
12. Uganda Bureau of Statistics. National Population Census and Housing. The State of Uganda Population Report 2001. Kampala: Bureau of Statistics, 2002. [ Links ]
13. Capital Assets and Depreciation Policy: Financial Statement. Windham, New Hamsphire: Town of Windham, 2005. www.windhamnewhampshire.com/ govt/policies/CapitalAssetsPolicy.pdf (accessed 11 July 2005). [ Links ]
14. Macintyre CR, Plaant AJ, Hendrie D. The cost-effectiveness of the evidence based guidelines and practice for screening and prevention of tuberculosis. Health Econ 2000; 9: 411-421. [ Links ]
Accepted 12 June 2009.
* Corresponding author: Andrew Livex Okwi (livexo@yahoo.co.uk)














