Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.99 no.12 Pretoria Dez. 2009
ORIGINAL ARTICLES
Anaemia among patients with congestive cardiac failure in Uganda – its impact on treatment outcomes
Julius Kabbali KuuleI, *; Emmanuel SerembaII; Juergen FreersIII
IMB ChB, MMed (Internal Medicine). Faculty of Medicine, Gulu University, Uganda
IIMB ChB, MMed (Internal Medicine). Mulago Hospital and Makerere University College of Health Sciences, Kampala, Uganda
IIIMB ChB (Hons), MMed (Internal Medicine), MSc. Makerere University College of Health Sciences, Kampala
ABSTRACT
BACKGROUND: Anaemia increases morbidity and mortality in patients with congestive cardiac failure (CCF). Few studies have examined the prevalence of anaemia and its impact among patients with CCF in sub-Saharan Africa. We assessed the prevalence of anaemia and its influence on treatment outcome in patients with CCF attending a large referral hospital in Kampala, Uganda.
METHODS: Echocardiography was done and haemoglobin levels were determined in 157 patients with CCF admitted to Mulago Hospital. The patients were followed up for 2 weeks and their treatment outcome was recorded.
RESULTS: Of the 157 patients, 101 (64.3%) had anaemia (mean haemoglobin concentration <11.9 g/dl for women and <12.9 g/dl for men) at admission. Increasing age and hypertensive heart disease were significantly associated with anaemia (odds ratio (OR) 2.92, confidence interval (CI) 1.41 - 6.05, p<0.01 and OR 0.31, CI 0.13 - 0.74, p< 0.01, respectively). In-hospital mortality at the end of the 2 weeks of treatment was 10.2% and was significantly higher among the anaemic patients than their non-anaemic counterparts (OR 4.9, CI 1.07 - 22.35, p<0.03). The mean duration of in-hospital stay was 7.5 (standard deviation 3.4) days. This did not differ significantly between anaemic and non-anaemic patients.
CONCLUSION: The prevalence of anaemia among patients with CCF attending Mulago Hospital was high. Anaemia in these patients was significantly associated with mortality by the end of 2 weeks of treatment.
Congestive cardiac failure (CCF) is a lethal end-stage complication of cardiovascular diseases of which the causes include hypertension, rheumatic heart disease and cardiomyopathies, and has reached epidemic proportions.1 CCF affects 2% of the world's population, and its prevalence increases with age.2 Cardiovascular diseases are an increasing cause of morbidity and mortality, and in 1990 two-thirds of all deaths from cardiovascular disease occurred in the developing world.1
The burden of CCF is expected to rise as medical and surgical interventions improve the prognosis of the underlying cardiac diseases, and as the proportion of elderly people increases.3 Epidemiological studies indicate a poor outcome with regard to progression and survival, with a mortality rate for patients with CCF being 4 - 8 times that of the general population of the same age.2The 1-year mortality rate for mild CCF is about 15%, increasing to 50% for severe disease.4 Long-term survival of patients with CCF is poor, with only 25% of men and 38% of women still alive at 5 years.5 Effective management depends on knowledge of factors that determine morbidity and mortality in this patient subset. Anaemia is a common co-morbidity in CCF and has important implications for management and outcome.
In developed countries short-term management outcomes include a mean in-hospital mortality rate of 6.4% and an average length of stay in hospital of 7.4 days.6 There are limited data on long- and short-term outcomes for CCF patients in sub-Saharan Africa.
Methods
This was a cross-sectional study in which patients admitted to the medical wards of Mulago national referral hospital, Kampala, Uganda, were recruited. The study was approved by the Makerere University College of Health Sciences Research and Ethics Committee and the Uganda National Council for Science and Technology. Consecutive patients admitted to the wards were approached and briefed about the study. Informed consent was obtained from participants. Minor children (N=10, ages 13 - 17) were recruited after obtaining assent from them and consent from their legal guardians. Participants were followed up prospectively for 2 weeks to determine the outcome of their treatment. Unconscious patients were excluded.
A diagnosis of CCF was made using the Framingham criteria and classified according to severity using the New York Heart Association (NYHA) grading system. In addition to echocardiography and measurement of the haemoglobin (Hb) concentration, socio-demographic data and information on risk factors for cardiac disease (including rheumatic fever, cardiomyopathies, previous hospitalisation and cardiac-related surgery) were collected by questionnaire. Echocardiography was done using a digital Sonoace 9000 Echo Copier (Medison Co. Ltd, Serial SA 5500 100 – 120/200 – 00501), while Hb was measured on a haemolyser (CELL-DYN 1700 system 1995).
The prevalence of anaemia was calculated as the proportion of men and women who had low Hb levels (<11.9 g/dl for women and <12.9 g/dl for men). Anaemia was categorised as mild (Hb 10 - 11.9 g/dl for women and 10 - 12.9 g/dl for men), moderate (Hb 7 - 9.9 g/dl for both genders) or severe (Hb <7 g/dl). Categorical variables including gender, causation of CCF and NYHA class were presented using frequencies and percentages and then cross-tabulated against the outcome variable (anaemia). Continuous variables such as age, urea, creatinine and fractional shortening were compared between the anaemic and non-anaemic patients using the independent-samples t-test for comparison of means. Levine's test for equality of variances and the significance level corresponding to the assumption of equal variance was used. The value of the t-statistic for the equality of means was used as a measure of strength of association. Multivariate logistic regression was used to determine strength of association between the presence of anaemia and other variables found to be significantly associated with anaemia at bivariate analysis.
Results
Patient characteristics
Of the 157 patients studied, 104 (66.2%) were female. The mean age was 45 years (range 13 - 99).
Underlying causation of CCF
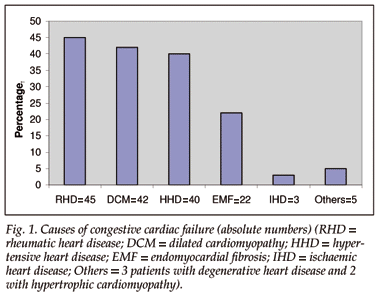
Rheumatic heart disease (28.2%), dilated cardiomyopathy (27.3%), hypertensive heart disease (25.1%) and endomyocardiofibrosis (EMF) (14.3%) were the most common underlying cardiac diseases, followed by degenerative valvular heart disease (1.9%), hypertrophic cardiomyopathy (1.3%) and ischaemic heart disease (1.9%), which was presumably due to coronary artery disease (Fig. 1). Most patients were admitted in severe CCF (NYHA classes III (59.9%) and IV (36.9%)). Only 5 (3.2%) were admitted in NYHA class II, and no patient was in NYHA class I.
Prevalence, severity and type of anaemia, and associations (Table I)
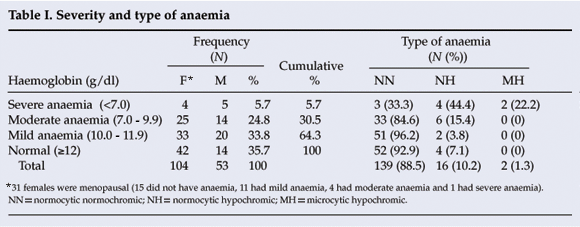
Anaemia was highly prevalent, occurring in 64.3% of the patients with CCF. The mean Hb was 11.2 g/dl (standard deviation (SD) 8.69). The prevalence of anaemia decreased along the scale from mild (31.2%) to severe (6.1%). The commonest type of anaemia was normocytic normochromic (88.2%).
Having hypertensive heart disease was significantly associated with anaemia (odds ratio (OR) 0.31, 95% confidence interval (CI) 0.13 - 0.74, p<0.01), as were increasing age (>50 years) (OR 2.92, CI 1.41 - 6.05, p<0.01) and raised creatinine (>133 µmol/l) (OR 0.17, 95% CI 0.08 - 0.37, p<0.01) and urea (>17.85 mmol/l) (OR 0.12, 95% CI 0.05 - 0.31, p<0.01).
Management outcomes
We examined mortality at the end of 2 weeks of hospitalisation and length of in-hospital stay.
The in-hospital all-cause mortality rate by the end of 2 weeks of treatment was 10.2%, with the mean number of days until death being 4.9 (SD 4.01) days. As expected, the greatest mortality occurred among patients with NYHA class IV, accounting for 68.2% of the overall mortality. Furthermore, 19.1% and 5.3% of patients in NYHA classes IV and III respectively died during the study period.
Looking at the causation of CCF, the greatest all-cause mortality occurred among patients with hypertensive heart disease (HHD), of whom 20.0% died (accounting for 50.0% of the deaths overall), followed by patients with rheumatic heart disease, of whom 11.1% died (31.2% of deaths overall). The odds of dying within 2 weeks were about 5 times higher in the anaemic group than in their non-anaemic counterparts, and this difference was statistically significant (OR 4.9, CI 1.07 - 22.35, p<0.01).
There was no significant difference in duration of hospitalisation between the anaemic and non-anaemic patients. The average length of stay in the ward was 7.5 (SD 3.4) days, with higher NYHA class being associated with longer stay; NYHA IV patients averaged 8.5 days, NYHA III patients 7.1 days and NYHA II patients 2.2 days.
Discussion
We found that 64.3% of CCF patients admitted to the Mulago national referral hospital medical wards had anaemia, which is comparable to studies done elsewhere.7-9 However, most of the studies on anaemia in patients with CCF available for comparison were conducted in developed countries, and there have been few African studies.10-12
A study in Israel found a 52.4% prevalence of anaemia in CCF patients.7 This study was similar to ours with regard to methodology and the NYHA classes (IV and III) of study participants. Their patients were generally older, however (mean age 76 years), and the underlying cause of CCF was predominantly ischaemic heart disease (77.1%), in contrast to our patients, who had a mean age of 45 years and in whom rheumatic heart disease, dilated cardiomyopathy and hypertensive heart disease accounted for over 82% of underlying causes. Our findings also contrast with those of Inglis et al. in South Africa,10 who found the prevalence of anaemia in patients with CCF to be 10%. Although the underlying causes of CCF in the South African cohort compare fairly well with the Ugandan cohort, the South African study clearly recruited patients with relatively milder degrees of cardiac failure (NYHA class II 42%, class III 33%), in which anaemia is generally less frequent; moreover, the patients were first-time attendees at the cardiology unit. The underlying mechanisms of anaemia in cardiac failure mean that first-time attendees may not have a high prevalence of anaemia because chronic cytokine release might not have had the critical cumulative effect leading to anaemia of chronic disease.
The overall prevalence of anaemia in CCF patients in a New York study was 61.0%,8 a finding similar to ours, as was the mean age of their patients (51.5 years). In our study, 72.4% of patients in NYHA class IV had anaemia compared with 57.4% in NYHA class III, which correlates with prevalences of anaemia of 79.1% and 52.6% in NYHA classes IV and III, respectively, in a study in Israel.9 This similarity in prevalence is surprising given the differences in mean age of the patients in the two studies – the patients in Israel were on average 70 years old. We found that the prevalence of anaemia in CCF increased with increasing patient age, as has been observed by others.13 This may be due to increasing co-morbidities with ageing.
On analysis of the causes of CCF, there was a statistically significant association between anaemia and hypertensive heart disease (OR 0.31, CI 0.13 - 0.74, p<0.01) that might reflect some underlying relative degree of hypertensive renal disease with concomitant relative erythropoietin deficiency in addition to the general mechanisms of anaemia causation in CCF.14
While the prevalence of anaemia in our study is comparable to rates in the developed countries, the underlying causes of CCF are not. This may imply that the anaemia in our patients is occurring as an effect of CCF per se rather than an effect related to the underlying cause of the CCF. However, we might be dealing with some co-morbidities that have still to be identified.
Valvular heart diseases, EMF, dilated cardiomyopathy and hypertensive heart disease (probably because blood pressure control is poor) remain significant causes of CCF in our patients. This compares well with a South African study11 in which it was found that hypertensive heart disease and dilated cardiomyopathy are the major underlying causes of CCF, with few cases of ischaemic heart disease. However, we seem to have higher numbers of patients with CCF resulting from rheumatic heart disease and EMF, a finding attributed by some15 to differences in traditional diet, socio-economic status and unidentified infections (eosinophilia). Few patients (1.9%) had ischaemic heart disease ascribed to coronary artery disease, although we did not do coronary artery angiography. Similarly, Sliwa12 found only a small number of patients with ischaemic heart disease (9%) in their cardiac failure cohort, whereas ischaemic heart disease is the predominant underlying cause of CCF in developed societies.7,9 With epidemiological change the underlying causes of CCF in our setting will certainly continue to evolve.
Mechanisms of anaemia in CCF
Several mechanisms (Fig. 2) have been proposed to explain the occurrence of anaemia in CCF, and it is probable that individual patients have varying combinations of these. CCF plays an important role in the causation of anaemia, mainly due to the depressant effects of tumour necrosis factor alpha (TNFα),16 which is secreted from the damaged myocardium.9

The TNFα interferes with erythropoietin-induced bone marrow stimulation, reduces the production of erythropoietin in the kidneys and interferes with the release of iron from the reticulo-endothelial system, depriving the bone marrow of this essential element for the production of Hb.16 Furthermore, the renal failure/damage that inevitably accompanies cardiac failure decreases erythropoietin production. In our study, where 95% of the participants had a structural cardiac disease (rheumatic heart disease, dilated cardiomyopathy, hypertensive heart disease or EMF, which lead to structural changes in the myocardium) as the underlying cause of CCF, this mechanism may have contributed significantly to the high prevalence of anaemia.
The reduced renal blood flow caused by CCF can lead to renal hypoxia, which can activate the renin-angiotensin axis and other growth factors, leading to glomerular and medullary damage and consequently proteinuria.17,18 In a vicious circle, proteinuria can further damage the renal tubules and reduce production of erythropoietin.19 Some degree of erythropoietin resistance occurs among CCF patients as a result of elevated plasma levels of inflammatory cytokines.20
In patients with chronic cardiac diseases, sequestration of iron in macrophages (consequent to chronic diseases) makes this nutrient unavailable for haematopoiesis. This would have applied to a large proportion of our patients and could have contributed to the high prevalence of anaemia.21 In addition, poor nutritional intake and gastro-intestinal malabsorption, which are common among patients with advanced CCF, could have played a role.22
On top of the mechanisms suggested above, the anaemia in cardiac failure patients may be due to haemodilution, which in one study was responsible for 46% of the anaemia.8 However, we did not assess patients for haemodilution.
Anaemia in patients with cardiac disease can also be iatrogenic, caused by drugs such as angiotensin-converting enzyme inhibitors and aspirin, which are thought to inhibit both erythropoietin production in the kidneys and its utilisation in the bone marrow and also cause gastro-intestinal bleeding.23,24
It is also important to note that anaemia is endemic in our community, with prevalences of 60.1% in children, 30.2% in women and 18.1% in men.25 Whether our high prevalence of anaemia reflects the generally high prevalence in the community requires further investigation.
Mortality
The all-cause in-hospital mortality rate in our study was 10.2%. This is comparable to mortality rates in other studies. A study in California reported an in-hospital mortality rate of 6.9%.6 Our rate may be slightly higher because our patients tend to present late with advanced disease. Furthermore, differences in the underlying cause of CCF may be partly responsible for differences in mortality rates. We established that the odds of dying in the first 2 weeks after admission for CCF patients with anaemia are 5 times higher than for CCF patients without anaemia. Early detection and appropriate management of anaemia in patients with CCF may improve their outcome.
Hospital stay
The mean length of hospital stay in our study was 7.5 days. The study was designed to follow up patients for a maximum of 2 weeks in hospital, yet some patients stayed longer than this. Our calculated mean duration of hospitalisation was therefore probably lower than would have been the case had patients been followed up until discharge. Nordyke and James found that their CCF patients spent a mean of 7.4 days in hospital, those with anaemia staying longer (p<0.01).6 In a Spanish study by Formiga, on the other hand, the mean length of stay for patients admitted with new-onset cardiac failure was 10 days.26 We suggest that this longer stay may in part be due to the time-consuming diagnostic work-up and counselling necessary for new patients.
Our findings suggest that if we were to measure the Hb concentration on admission of every patient with CCF and treat anaemia if present, we could provide better care for these patients and reduce in-hospital mortality.
Limitations
We were unable to follow up all patients until discharge and therefore cannot come to accurate conclusions on the effect of anaemia on length of hospital stay. However, data obtained during the 2-week follow-up are sufficient for us to conclude that anaemia in CCF signifies an unfavourable outcome.
Recommendations
A long-term follow-up study in a setting similar to ours is recommended. Studies looking at optimal treatment of anaemia in CCF urgently need to be carried out in sub-Saharan settings.
This study was partly funded by the KULIKA charitable trust and Roche, Uganda. We thank the patients who participated.
Conflict of interest. None.
References
1. Srinath RK, Salim Y. Emerging epidemic of cardiovascular diseases in developing countries. Circulation 1998; 97: 596-601. [ Links ]
2. Kinnel W, Thom T. Changing epidemiological features of cardiac failure. Br Heart J 1994; 72: S3-S9. [ Links ]
3. Norman S, Doughty R. Epidemiology of heart failure and left ventricular dysfunction. Lancet 1998; 352: suppl 1, 3-7. [ Links ]
4. Mosterd A. The prognosis of heart failure in the general population. The Rotterdam study. Eur Heart J 2001; 22: 1318-1327. [ Links ]
5. Ho KKL, Levy D. Survival after the onset of congestive heart failure in the Framingham heart study subjects. Circulation 1993; 88: 107-115. [ Links ]
6. Nordyke R, James W. Impact of anemia on hospitalization time, charges and mortality in patients with heart failure. Value in Health 2004; 7(4): 464-471. [ Links ]
7. Wexler D, Silverberg D, Sheps D, et al. Prevalence of anemia in patients admitted to hospital with a primary diagnosis of congestive heart failure. Int J Cardiol 2004; 96: 79-87. [ Links ]
8. Androne AS, Mancini DM. Haemodilution is common in patients with advanced heart failure. Circulation 2003; 107: 226-229. [ Links ]
9. Silverberg DS, Wexler D, Blum M, et al. The use of subcutaneous erythropoietin and intravenous iron for the treatment of the anemia of severe congestive heart failure improves cardiac and renal function and functional cardiac class and markedly reduces hospitalizations. J Am Coll Cardiol 2000; 35: 1737-1744. [ Links ]
10. Inglis SC, Stewart S, Sliwa K, et al. Anaemia and renal function in heart failure due to idiopathic dilated cardiomyopathy. Eur J Heart Fail 2007; 9: 384-390. [ Links ]
11. Stewart S, Wilkinson D, Sliwa K, et al. Predominance of heart failure in the Heart of Soweto study cohort: Emerging challenges for urban African communities. Circulation 2008; 118: 2360-2367. [ Links ]
12. Sliwa K, Wilkinson D, Stewart S, et al. Spectrum of heart diseases and risk factors in a black urban population in South Africa (the Heart of Soweto study). Lancet 2008; 371: 915-922. [ Links ]
13. Spencer FA. Twenty year trends (1975 - 1995) in incidence, in-hospital and long-term death rates associated with heart failure complicating AMI. J Am Coll Cardiol 1999; 34(5): 1378-1387. [ Links ]
14. Ezekowitz AJ, Armstrong PW. Anemia is common in heart failure and is associated with poor outcomes. Circulation 2003; 107: 223. [ Links ]
15. Freers J, Masembe V, Schmauz R, Mayanja H. Endomyocardial fibrosis syndrome in Uganda. Lancet 2000; 355: 1994-1995. [ Links ]
16. Means RT. Advances in the anemia of chronic disease. Int J Hematol 1999; 70: 7-12. [ Links ]
17. Fine LG, Norman JT. Progressive renal disease: The chronic hypoxia hypothesis. Kidney Int 1998; 53: S74-S78. [ Links ]
18. Yoshida H, Shimada E. Mesangiolytic glomerulopathy in severe congestive heart failure. Kidney Int 1998; 53: 880-891. [ Links ]
19. Jafar TH, Stark PC, Schmid CH, et al. Proteinuria as a modifiable risk factor for the progression of non-diabetic renal disease. Kidney Int 2001; 60: 1131-1140. [ Links ]
20. Torre-Amione G, Bozkurt B, Deswal A, Mann DL. An overview of tumor necrosis factor alpha and the failing human heart. Curr Opin Cardiol 1999; 14: 206-210. [ Links ]
21. Ludwiczek S, Weiss G. Cytokine-mediated regulation of iron transport in human monocytic cells. Blood 2003; 101: 4148. [ Links ]
22. Parsi A, Kleber F. Anemia in heart failure: Its diagnosis and management. Eur J Heart Fail 2003; 5: 3-4. [ Links ]
23. Macdougall IC. The role of ACE inhibitors and angiotensin II receptor blockers in the response to erythropoietin. Nephrol Dial Transplant 1999; 14: 1836-1841. [ Links ]
24. Silagy CA, McNeil J, Donnan G, Tonkin M, Worsam B, Campion K. Adverse effects of low-dose aspirin in a healthy elderly population. Clin Pharmacol Ther 1993; 54: 84-89. [ Links ]
25. Uganda Bureau of Statistics (UBOS) and Macro International Inc. Uganda Demographic and Health Surveys 2006. Kampala, Uganda, and Calverton, Md: Uganda Bureau of Statistics and Macro International Inc., 2007. [ Links ]
26. Formiga F. Anemia in new-onset congestive heart failure patients admitted for acute decompensation. Eur J Intern Med 2006; 17(3): 179-184. [ Links ]
Accepted 26 August 2009.
* Corresponding author: J K Kuule (jkkuule2000@yahoo.com)














