Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.99 no.12 Pretoria Dez. 2009
SCIENTIFIC LETTERS
First do no harm: addressing respiratory morbidity in the newborn and child following elective caesarean section before 39 weeks' gestation
J SmithI, *; R J AlexanderII
IMB ChB, MMed, PhD. Division of Neonatology, Department of Paediatrics, Stellenbosch University and Tygerberg Children's Hospital, and Panorama Medi-Clinic Neonatal Intensive Care Unit, Parow
IIMB ChB, DCH (SA), MMed, FCPaed (SA). Panorama Medi-Clinic Neonatal Intensive Care Unit, Parow
To the Editor: Globally, caesarean section (CS) rates are increasing and vary between 18% and 31%,1,2 but between 65% and 82% in some countries with private health care.3,4
A CS performed for the right indication is in the best interest of the mother and/or baby. However, the indications for an elective CS at or near term remain controversial and vary widely. CS on request before 39 weeks' gestation and/or those performed for physician convenience or after incorrect interpretation of dates are of special interest. These elective CS criteria are unlikely to be in the best interests of the unborn child, mother, or family if performed too early. The interplay between a maternal request based on patient autonomy, (consumer) choice or convenience and physician threshold for choosing it before the due date remains a concern. Our experience is that these 'reasons' are rarely accurately recorded in the clinical notes, which often reflect unconvincing medical reason(s) for performing early CS. However, one cannot ignore the trend that an elective CS following a maternal request is regarded as a valid option in some developed countries and is often the major factor influencing the mode of delivery,5-7 including a belief that its risks for healthy women are so low that CS is a reasonable elective childbirth option.8
To determine the respiratory morbidity related to CS, we reviewed our own experience with infants of a gestation of >37 weeks, who required admission to a private neonatal intensive care (NIC) unit over a 6-year period. We then extrapolated our own and other results to create a scenario relevant to South Africa.
The discussion focuses on after-birth physiological adaptation of the full-term newborn after CS and its poorly recognised associated respiratory neonatal and post-neonatal morbidity. Evidence-based recommendations are made to lower this health care burden.
Study population and methods
We reviewed the hospital records of consecutively admitted infants to the Panorama NIC unit between January 2003 and December 2008. The review focused on those of >37 weeks' gestation with respiratory-related conditions. These diagnoses included 'respiratory distress', 'respiratory distress syndrome (RDS)', 'persistent pulmonary hypertension of the newborn (PPHN)', 'transient tachypnoea of the newborn (TTN)', 'pneumothorax', or combinations thereof. Infants with a diagnosis of meconium aspiration or pneumonia were excluded. Data on invasive and/or non-invasive ventilatory support were retrieved. Statistical analysis was performed using SSC-Stat version 2.12. A p-value <0.05 was accepted as significant.
Results
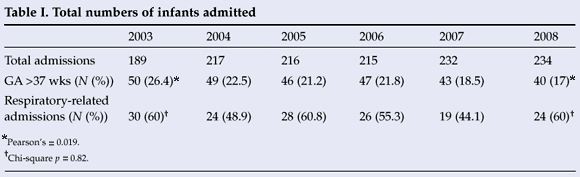
Of the 1 303 infants admitted, 275 (21.1%) were of >37 weeks' gestation (Table I). Of the 275 infants who were of >37 weeks' gestation, 135 (49%) were admitted with one or more of the respiratory-related diagnoses. Respiratory distress or RDS was diagnosed in 75.6% and TTN in 24.4%. Significant PPHN was associated with either RDS or TTN in 15.5%, and a pneumothorax occurred in 7.4%. A total of 79 (58.5%) with a respiratory-related diagnosis required ventilation support; of them, 31 (22.9%) were ventilated and 48 (35.5%) received nasal continuous positive airway pressure (NCPAP). Surfactant was administered in 10 (12.6%), and 13 (16.4%) infants were treated with inhaled nitric oxide (62% of infants diagnosed with PPHN). Despite a 20% reduction in the number of infants admitted with a gestation exceeding 37 weeks between 2003 and 2008 (Pearson's, p=0.019), the number of infants admitted with respiratory-related diagnoses in this category did not reach significance (chi-square; p=0.82). After 2005, a trend towards fewer infants admitted with respiratory-related diagnoses was observed (p=0.06). Of the 135 infants admitted with respiratory-related diagnoses, 96% were delivered by CS.
Discussion
After birth, a newborn faces a big challenge to rapidly adjust its physiology from the intrauterine milieu with fluid-filled lungs, to an environment in which the lungs are filled with air. CS infants are deprived of natural adaptive physiological responses.1,6,9,10 Postnatal adaptation and development of neonates could be different due to the birth method and these effects may last throughout adulthood.6,11-14 Owing to altered physiological responses, CS babies have lower 1-minute Apgar scores, higher need for resuscitation in the delivery room and at least 5 times higher requirements for ventilatory support.11,15-17
Palme-Kilander et al. measured various parameters during the first 5 minutes after birth in healthy newborn infants after vaginal delivery or CS.18 There was a clear difference in gas exchange between groups, the VCO2 and VO2 of vaginally delivered infants remained higher, while the VCO2 and VO2 of CS neonates declined to levels lower than the basic metabolic rate of 5 - 6 ml/kg/minute. Inadequate lung aeration with decreased functional residual capacity and hypoventilation could have been responsible for these differences. A delay in establishing final lung volumes of up to 24 hours was described in babies born without labour or passage through the birth canal.19 Therefore, compared with vaginal deliveries, there is an increased likelihood of delivery room (DR) morbidity associated with elective CS at or near term, necessitating the presence of trained staff to stabilise the condition of the neonate after delivery.16,17
Prior to 39 weeks' gestation, and in the absence of risk factors, an elective CS increases iatrogenic prematurity and respiratory morbidity,1,9,11 Studies show significantly increased odds (2- to 5-fold) of respiratory morbidity of near-term infants after elective CS compared with babies born vaginally.20,21 More specifically, the respiratory morbidities assessed included RDS, TTN and PPHN. Stratified by gestational age, the risk decreased from 14-fold higher at 37 completed weeks of gestation to 3.5-fold higher at 39 completed weeks of gestation.20,22 Respiratory-related morbidity determined from more than 24 000 newborn births, including near-term neonates (>37 weeks' gestation) born by elective CS without a trial of labour, clearly showed higher occurrence of RDS, TTN, NIC admission, oxygen therapy and ventilator care, compared with successful vaginal birth.9 TTN is usually considered to be benign and self-limiting; however, both our and previous studies describe severe respiratory failure after elective CS resulting in need for ventilation, including high-frequency oscillation, surfactant and inhaled nitric oxide.23 The timing of elective CS influences the pneumothorax risk, with a reported decrease in the incidence from week 37 to 37+6 onward.24
Regarding cardiovascular adaptation, a decrease was shown in cardiac output and heart rate during the first 48 hours of life in CS and vaginally delivered infants. In spite of increased catecholamine levels in the vaginally delivered infants, differences between the groups were marginal.25 Likewise, despite higher umbilical arterial epinephrine and norepinephrine concentrations in the vaginally delivered infants compared with CS-delivered infants, similar findings were reported for left ventricular output and its regional distribution between CS and vaginally delivered neonates.26 Therefore, the capacity of infants delivered by CS to tolerate cardiovascular changes during the early neonatal period is comparable with those delivered vaginally, even though there are significant differences in their catecholamine surge. During the early neonatal period, significant differences exist between CS-delivered and vaginally delivered babies in lung adaptation despite there being no real difference in their respective cardiovascular situations.
We found no significant decrease in the immediate neonatal respiratory-related morbidity experienced by infants delivered by CS after >37 weeks' gestation. Others also found that the electively delivered term infant is at significant risk of respiratory morbidity which is potentially preventable if evidence-based practices are followed.9 In South Africa, approximately 750 000 babies are delivered each year at term gestation. An estimated 15% and 85% of them are delivered in private and public facilities, of whom some 60% and 25% respectively are delivered by CS.4 If an elective CS was performed for a non-medical reason in an estimated 20% of the CS deliveries after 37 weeks' gestation, the expected number of elective CS-delivered babies who present annually to private and public neonatal units with respiratory-related conditions would be in the order of 6 000 and 15 000, respectively. These figures (~2.8% of term deliveries) highlight an area of health care that requires much more attention to improve neonatal care and long-term outcome. Long-term effects following CS delivery are increasingly reported. Neonatal immune responses can be altered and the risk of atopy increased. It is therefore plausible that the mode of delivery might have contributed to the global rise in asthma. Children born by CS have a higher risk of asthma than those born vaginally, particularly children of allergic parents. CS increases the risk for sensitisation to common allergens in children with non-allergic parents.27 Among children with a parental history of asthma or allergies and born by CS, there was a twofold higher odds of atopy compared with those born vaginally, and birth by CS was significantly associated with increased odds of allergic rhinitis, but not with asthma.28 A systematic review found that the risk of developing IgE-mediated sensitisation to food allergens is increased among children delivered by CS compared with those delivered vaginally.29
The association between elective CS and respiratory problems could be because labour is important for neonatal adaptation. The squeeze through the birth canal triggers a surge of hormones at birth, particularly catecholamines, and these, particularly adrenaline, inhibit lung liquid secretion and stimulate its absorption, enhancing aeration of the lungs.6 A randomised controlled trial found that infants delivered by elective CS (37 - 39 weeks' gestation) whose mothers were given intravenous terbutaline had lower respiratory morbidity, better lung function and higher adrenaline and blood glucose concentrations compared with a control group.30 These findings supported the premise that stimulation of beta-adrenoceptors with adrenoceptor agonists before an elective CS may promote postnatal respiratory and metabolic adaptation. Stutchfield et al. showed that antenatal betamethasone administered within 48 hours of delivery and delaying non-urgent elective CS until 39 weeks both reduced admissions to neonatal units.31 The benefits of antenatal steroids persisted until 39 weeks and antenatal betamethasone reduced the incidence of TTN from 4% of elective CS to 2.1% (decrease of 47%) and that of RDS from 1.1% to 0.2% (decrease of 43%).
Although few South African figures exist regarding respiratory-related morbidity after elective CS, we believe that CS performed before 38 completed weeks of gestation is a child health issue.32 It is therefore pertinent to debate how the elective CS at or near term could be more appropriately managed and the number of cases admitted to neonatal units thus lowered. One step could be to clarify or debate the importance of maternal/parental choice about CS for non-medical reasons. If maternal autonomy is accepted in this regard, our view, shared by others, is that there should be agreement on choice as a valid reason for performing an elective CS only in pregnancies without prenatally identified risk factors, and that this is recorded. Moreover, whenever possible, CS should not be performed earlier than at 38 completed weeks of gestation, i.e. before 39+0. If the exact dates are uncertain or maternal choice is exercised, there should be proper consultation, informing the mother of the probable benefits of prenatal steroid treatment and risks that CS may pose to her and, more importantly, to her offspring. She should be informed of the risk of asthma for her child, especially if parents have a history of allergy or asthma.27 Non-medical CS must be clinically evaluated in each case. With current evidence for long-term adverse child health outcomes, it also presents an ethical issue: How much can consumer autonomy override medical evidence for an adverse long-term outcome in the child? Chronic illness and disability may unwittingly be fostered by uncontrolled non-medical indications for CS.
References
1. Ramachandrappa A, Jain L. Elective cesarean section: Its impact on neonatal respiratory outcome. Clin Perinatol 2008, 35: 373-393. [ Links ]
2. Belizán JM, Althabe F, Barros FC, Alexander S. Rates and implications of caesarean sections in Latin America: ecological study. BMJ 1999; 319: 1397-1400. [ Links ]
3. Richard D. The impact of elective caesarean sections on NICU admissions of term and near-term infants. Vermont Oxford Network Symposium, Somerset West, Cape Town, 17-19 October 2008. [ Links ]
4. Bateman C. Rendering unto Caesar? S Afr Med J 2004; 94: 800-802. [ Links ]
5. Eftekhar K, Steer P. Caesarean section controvery. Women choose caesarean section. BMJ 2000; 320: 1073. [ Links ]
6. Grisaru S, Samueloff A. Primary nonmedically indicated cesarean section ('section on request'): evidence based or modern vogue? Clin Perinatol 2004; 31: 409-430. [ Links ]
7. Wilkinson C, McIlwaine G, Boulton-Jones C, Cole S. Is a rising caesarean section rate inevitable? Br J Obstet Gynaecol 1998; 105: 45-52. [ Links ]
8. National Institute of Health state-of-the-science conference statement: cesarean delivery on maternal request. Obstet Gynecol 2006; 107: 1386-1397. [ Links ]
9. Jain L, Eaton DC. Physiology of fetal lung fluid clearance and the effect of labor. Sem Perinatol 2006; 30: 34-43. [ Links ]
10. Levine EM, Ghai V, Barton JJ, Strom CM. Mode of delivery and risk of respiratory diseases in newborns. Obstet Gynecol 2001; 97: 439-442. [ Links ]
11. Zanardo V, Simbi AK, Franzoi M, Solda G, Salvadori A, Trevisanuto D. Neonatal respiratory morbidity risk and mode of delivery at term: influence of timing of elective caesarean delivery. Acta Paediatr 2004; 93: 643-647. [ Links ]
12. Morrison J, Rennie JM, Milton PJ. Neonatal respiratory morbidity and mode of delivery at term: influence of timing of elective cesarean section. Br J Obstet Gynaecol 1995; 102: 101-106. [ Links ]
13. Madar J, Richmond S, Hey E. Surfactant-deficient respiratory distress after elective delivery at term. Acta Paediatr 1999; 88: 1244-1248. [ Links ]
14. Kim HR, Jung KY, Kim SY, Ko KO, Lee YM, Kim JM. Delivery modes and neonatal EEG: spatial pattern analysis. Early Hum Dev 2003; 75: 35-53. [ Links ]
15. Annibale DJ, Hulsey TC, Wagner CL, Southgate WM. Comparative neonatal morbidity of abdominal and vaginal deliveries after uncomplicated pregnancies. Arch Paediatr Adolesc Med 1995; 149: 862-867. [ Links ]
16. Press S, Tellechea C, Pregen S. Cesarean delivery of full-term infants: Identification of those at risk for requiring resuscitation. J Pediatr 1985; 106: 477-479. [ Links ]
17. Hogston P. Is a paediatrician required at caesarean section? Eur J Obstet Gynecol Rep Biol 1987; 26: 91-93. [ Links ]
18. Palme-Kilander C, Tunell R, Chiwei Y. Pulmonary gas exchange immediately after birth in spontaneously breathing infants. Arch Dis Child 1993; 68: 6-10. [ Links ]
19. Lee S, Hassan A, Ingram D, Milner AD. Effects of different modes of delivery on lung volumes of newborn infants. Pediatric Pulmonol 1999: 27: 318-321. [ Links ]
20. Hansen AK, Wisborg K, Uldbjerg N, Hendriksen TB. Elective caesarean section and respiratory morbidity in the term and near-term neonate. Acta Obstet Gynecol 2007; 86: 389-394. [ Links ]
21. Hansen AK, Wisborg K, Uldbjerg N, Hendriksen TB. Risk of respiratory morbidity in term infants delivered by elective caesarean section: cohort study. BMJ 2008; 336 (7635): 85-87. [ Links ]
22. Riskin A, Abend-Weinger M, Riskin-Mashiah S, Kugelman A, Bader D. Cesarean section, gestational age, transient tachypnea of the newborn: timing is the key. Am J Perinatol 2005; 22: 377-382. [ Links ]
23. Keszler M, Carbone MT, Cox C, Schumacher RE. Severe respiratory failure after elective repeat cesarean delivery: a potentially preventable condition leading to extracorporeal membrane oxygenation. Pediatrics 1992; 89: 670-672. [ Links ]
24. Zanardo V, Padovani E, Pittini C, Doglioni N, Ferrante A, Trevisanuto D. The influence of timing of elective cesarean section on risk of neonatal pneumothorax. J Pediatr 2007; 150: 252-255. [ Links ]
25. Hirsimäki H, Kero P, Ekblad H, Scheinin M, Saraste M, Erkkola R. Mode of delivery, plasma catecholamines and Doppler-derived cardiac output in healthy term newborn infants. Biol Neonate 1992; 61: 285-293. [ Links ]
26. Agata Y, Hiraishi S, Misawa H, et al. Hemodynamic adaptations at birth and neonates delivered vaginally and by Cesarean section. Biol Neonate 1995; 68: 404-411. [ Links ]
27. Roduit C, Scholtens S, De Jongste JC, et al. Asthma at 8 years of age in children born by caesarean section. Thorax 2009; 64: 107-113. [ Links ]
28. Pistiner M, Gold DR, Abdulkerim H, Hoffman E, Celedón JC. Birth by cesarean section, allergic rhinitis, and allergic sensitization among children with a parental history of atopy. J Allergy Clin Immunol 2008; 122: 274-279. [ Links ]
29. Koplin J, Allen K, Gurrin L, Osborne N, Tang MLK, Dharmage S. Is caesarean delivery associated with sensitization to food allergens and IgE-mediated food allergy: A systematic review. Pediatr Allergy Immunol 2008; 19: 682-687. [ Links ]
30. Eisler G, Hjertberg R, Lagercrantz H. Randomised controlled trial of effect of terbutaline before elective caesarean section on postnatal respiration and glucose homeostasis. Arch Dis Child Fetal Neonatal Ed 1999; 80: F88-F92. [ Links ]
31. Stutchfield P, Whitaker R, Russell I, on behalf of the Antenatal Steroids for Term Elective Caesarean Section (ASTECS) Research Team. Antenatal betamethasone and incidence of neonatal respiratory distress after elective caesarean section: pragmatic randomized trial. BMJ 2005; 331; 662-668. [ Links ]
32. Rothberg AD, McLeod H. Private-sector caesarean sections in perspective. S Afr Med J 2005; 95: 257-260. [ Links ]
Accepted 6 May 2009.
* Corresponding author: J Smith (js7@sun.ac.za)














