Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.99 no.11 Pretoria Nov. 2009
ORIGINAL ARTICLES
Severe snakebites in northern KwaZulu-Natal: treatment modalities and outcomes
Darryl WoodI; Caroline WebbII; Jenine DeMeyerIII
IMB BCh, MPhil (Emerg Med), BPharm, DA, DipPEC. Ngwelezane Hospital, Empangeni, KwaZulu-Natal
IIMB BCh. Ngwelezane Hospital, Empangeni, KwaZulu-Natal
IIIMB BCh. Ngwelezane Hospital, Empangeni, KwaZulu-Natal
ABSTRACT
OBJECTIVE: We aimed to study the outcomes of severe snakebites in patients admitted to Ngwelezana Hospital in north-eastern KwaZulu-Natal, the seasonal variations, and the effectiveness and complications of antivenom.
DESIGN: A prospective observational outcomes study was conducted over one year (1 June 2007 to 31 May 2008). The study group was from the north-eastern KwaZulu-Natal region of South Africa, with a population of approximately 3 million people, and included all patients bitten by snakes and admitted to the Ngwelezana Hospital Emergency Medicine Unit (EMU). Departmental practice guidelines were documented and followed.
OUTCOME MEASURES: End-points for patient outcomes included transfer from the EMU to the ward, discharge home from the EMU, and follow-up of patients who required surgery or ICU care.
RESULTS: A total of 243 snakebite patients were recorded. The highest incidence was in the summer months; 46 (18.93%) patients experienced one or more severe complications; 29 (11.93%) patients received some form of definitive management in hospital; and 22 (9.05%) of the latter patients received antivenom. Antivenom was administered to more children than adults. Adverse reactions to antivenom were common: an allergic response occurred in 4 (15.4%) patients, and anaphylaxis in 6 (23.1%); the highest incidence occurred in the <10-year-old age group. No deaths were recorded.
CONCLUSIONS: Snakebites are common in the summer months in north eastern KwaZulu-Natal. Children are particularly vulnerable to snakebites and the effects of antivenom. Adverse reactions to antivenom are common. Severe snakebites that require antivenom should be managed in a hospital setting with advanced airway support. The syndromic approach to treatment is simple and effective.
Snakebites are common in the sub-tropical north-eastern region of KwaZulu-Natal. Several studies have investigated the epidemiology of snakebites in hospitals in rural KwazuluNatal.1-3 We aimed to observe outcomes of severe snakebites in patients admitted to Ngwelezana Hospital. Seasonal variations of snakebites and the effectiveness and complications of antivenom were assessed.
KwaZulu-Natal and Mpumulanga have the highest incidences of snakebites in South Africa, with 24 -34 per 100 000 people being bitten annually.4 Less than 10% of snakes in southern Africa are potentially lethal; the reported snakebite mortality rate is 1 -2%.4 The snakes responsible for severe bites in the study region include: forest cobra (Naja melanoleuca), M'Fezi or Mozambique spitting cobra (Naja mossambica), Egyptian cobra (Naja haje), black mamba (Dendroaspis polylepis), green mamba (Dendroaspis angusticeps), boomslang (Dispholidus typus), vine snake (Thelotornis capensis), puff adder (Bitis Arietans) and Gaboon viper (Bitis gabonica).2 The most commonly reported dangerous snakebites are from the M'fezi and puff adder,2 while the notorious black mamba causes lifethreatening neurotoxic envenomation.
Empangeni is a town in north-eastern KwaZulu-Natal. Ngwelezana Hospital is a 500-bed hospital situated in a semirural settlement outside Empangeni, and is the referral centre for 22 rural hospitals. It has an emergency medicine unit (EMU) where all snakebite patients are observed and treated.
Antivenom is manufactured by the South African Institute for Medical Research (SAIMR).5 It neutralises active snake venom and is the definitive management for severe snakebite envenomation. Polyvalent antivenom is effective against most snakes that are likely to cause life-threatening envenomation in southern Africa, except certain adders, the boomslang and the vine snake. The SAIMR has produced two monovalent antivenoms for the haemotoxic boomslang and the saw-scaled viper.
The indications for antivenom use are listed in Fig. 1. Studies in Eshowe and Durban showed that up to 41 -50% of patients experienced an adverse effect to antivenom use.1,6 Acute anaphylaxis (e.g. hypotension, bronchospasm) is not doserelated and typically occurs 1 -15 minutes after antivenom administration.5 Milder allergic reactions such as urticaria and tachycardia can also be experienced. Serum sickness (urticaria, polyarthritis, mild fever and lymphadenopathy) is usually delayed by up to 7 -12 days.5,7 Test dosing with antivenom is no longer recommended.8 Fig. 2 shows the guidelines for administering antivenom. The effectiveness of premedication with hydrocortisone, antihistamines and intramuscular adrenaline in reducing the risk of anaphylaxis is unknown.8
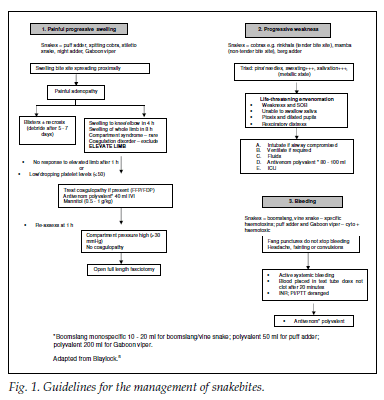

The dose of antivenom depends on the type and amount of venom injected rather than the weight of the patient. Children therefore receive the same amount of antivenom as adults. Further doses can be given and are titrated against the patient's response. Haemotoxic venoms tend to have a delayed onset.2
Practice guidelines adapted from Blaylock were used in this study.8
Methods
A prospective observational outcomes study from northeastern KwaZulu-Natal, with a population of approximately 3 million people, was conducted over 1 year (1 June 2007 to 31 May 2008). All patients who were bitten by snakes and admitted to the Ngwelezana Hospital EMU were documented. Departmental practice guidelines were followed (Fig. 1). All snakebite patients from the immediate local area (Uthungulu) were included. Only those with severe symptoms from outlying referral hospitals were accepted for admission to the EMU.
The data were collated daily by two doctors working in the EMU. Endpoints for patient outcomes were:
• transfer from the EMU to the ward
• discharge home from the EMU
• follow-up and documentation of patient outcomes with severe progressive symptoms requiring surgery or ICU care.
All patients with suspected snakebites admitted to the EMU were included and confirmed by cross-reference with the admission logbook of the unit.
Every patient was reviewed by a senior doctor (principal medical officer, chief medical officer or consultant) within 12 hours of admission. The management guidelines classify envenomation symptoms as painful progressive swelling, progressive weakness and bleeding.8 Patients with severe symptoms were observed for at least 12 hours, with regular documentation of the progression of swelling, neurological status, signs of bleeding and examination for compartment syndrome. Patients with an uneventful course were transferred to the ward. Patients with unconvincing snakebite symptoms (absence of fang marks or no local swelling) were discharged home following a period of observation and normal blood results. The causative snake was rarely identified as patients' herpetological knowledge was generally unreliable, and invariably the snake was not seen at the time of the bite.2
Severe complications associated with snakebites were categorised according to specific criteria:
1. Rapid progressive swelling. Swelling crossing 2 large joints within 4 hours or the entire limb by 8 hours following the snakebite. Snakebites that potentially compromised the airway were also included in this category.
2. Compartment syndrome. Following rapid progressive swelling, an increase in compartmental pressure resulting in microcirculatory arrest and vascular compromise to the limb.6 Snakebites usually cause subcutaneous oedema without raised compartment pressures.8 We did not measure compartmental pressures.
3. Haematological disorder. An international normalised ratio (INR) >1.5, haemoglobin <8.0 g/dl or platelet count <50×109/l.
4. Neurotoxicity. Symptoms suggestive of neurotoxicity from envenomation typically include drowsiness or a depressed level of consciousness, limb weakness, sweating, vomiting, inability to swallow saliva, ptosis, blurred or double vision, and respiratory muscle paralysis.4
Snakebites are usually categorised according to the primary action of their venom into cytotoxic, haemotoxic and neurotoxic. However, many snakes inject a variety of toxic compounds that may cause more than one type of syndrome.6 Patients with more than one type of severe complication had each one documented separately.
Antivenom administration was at the discretion of the consultant, according to departmental guidelines (Fig. 2). In all cases resuscitation equipment was on standby, in readiness for anaphylaxis. Hydrocortisone, antihistamine and adrenaline were given prior to the antivenom. The SAIMR polyvalent antivenom was diluted in saline and administered over a 15-minute period, with careful monitoring for allergic or anaphylactic responses.5 An initial standard dose of 4 vials of antivenom was used, regardless of age, and repeated as necessary. Patients with neurotoxic snakebites typically required higher doses of antivenom; endotracheal intubation was considered in these cases owing to the risk of respiratory muscle paralysis. Adverse reactions to antivenom administration were classified as either allergic (mild response, most commonly manifested as pruritus, skin weals and an associated tachycardia) or anaphylactic (severe, potentially life-threatening systemic reaction). Patients who became hypotensive or experienced bronchospasm were classified as having an anaphylactic reaction. Only acute reactions were documented. Serum sickness was not investigated owing to the inability to follow up patients after discharge from the EMU.
Results
A total of 243 snakebite patients were recorded; 29 (11.93%) were referred from peripheral hospitals within the Ngwelezana catchment area. Most snakebites were recorded in January (Fig. 3) (peak of summer, with average daily temperatures of around 32ºC and high humidity levels). The data show a rising trend in snakebites in the summer months. The most common snakebite population groups were children and young adults; 137 (56.4%) were <20 years old (Fig. 4).
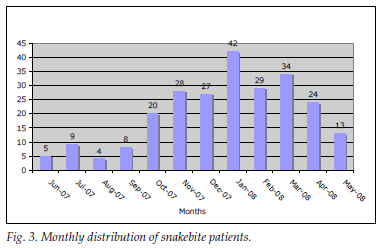
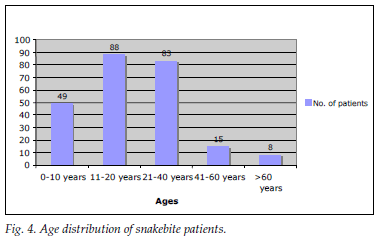
Most patients (81.1%) had mild symptoms. One or more severe snakebite complications were experienced by 46 (18.9%), as follows: haemotoxicity in 27 (58.7%); rapid progressive swelling in 18 (39.1%); compartment syndrome was diagnosed clinically in 4 (8.7%), but only one patient received a fasciotomy; and neurotoxicity in 3 (6.5%). All of them received antivenom. No patients died.
Twenty-nine (11.9%) patients received some form of definitive management; some received more than one, depending on complexity of the symptoms. Antivenom was administered to 75.9% of all patients who required definitive treatment; 22 (9.1%) received antivenom. Blood products (packed red cells, fresh-frozen plasma or platelets) were given in 6 cases; 3 required endotracheal intubation in the EMU (2 for severe neurotoxicity and 1 for anaphylaxis due to antivenom administration); and 11 (4.5%) required surgery including debridement of necrotic snakebite wounds, amputation of affected limbs or digits, or fasciotomy. All 4 cases of clinically diagnosed compartment syndrome received antivenom. One patient required a fasciotomy.
Antivenom administration
Twenty-two patients were given antivenom; 4 received antivenom at their referral hospitals before admission to the Ngwelezana EMU. Half of the patients given antivenom at Ngwelezana came from referring hospitals. Only patients with complicated snakebites that could not be managed at the peripheral referring hospitals were sent to Ngwelezana Hospital for further treatment.
Antivenom was administered to more children than adults: 15 (68.2%) patients receiving antivenom were <20 years old (0-10 years: 8, 11 - 20 years: 7, >20 years: 7). The most common indication for antivenom administration was rapid progressive swelling (14 (46.7%) of patients), with haemotoxicity in 8 (26.7%) coming next. All 4 cases of clinical compartment syndrome received antivenom. One patient received antivenom for venom-related shock. Many patients met the criteria for more than one indication for antivenom administration.
The mean time to antivenom administration was 16 hours after the bite; this delay can be attributed to:
• lack of transport in rural areas
• transfer of patients from referring hospitals
• patients seeking advice from traditional healers before going to hospital
• prolonged observation of symptom progression in the EMU.
Adverse reactions to antivenom were common; only 7 (31.8%) patients received antivenom without any adverse reaction. An allergic response occurred in 4 (15.4%) cases and anaphylaxis in 6 (23.1%) (Table I). Two patients were transferred to the ICU: an adult male with neurotoxic snakebite symptoms, and a young girl with rapid progressive swelling, compartment syndrome and reperfusion injury. One patient was transferred to a tertiary hospital following an anaphylactic reaction to the antivenom.
The highest percentage of adverse reactions to antivenom occurred in the 0 - 10-year age group, while most adults (85.7%) experienced no complications (Table I). Of the 8 patients under the age of 10 who received antivenom, only 2 (25%) had no adverse reaction. The highest proportion of anaphylactic reactions occurred in the 11 - 20-year age group (42.9%).
Discussion
Snakebites are common in KwaZulu-Natal. A study at a similar hospital in this region reported 282 severe bites over a 32-month period.1 As with similar studies in the region, the highest seasonal incidence was during the warm summer months.1,2
The EMU guidelines are based on those of Blaylock.8 These follow a syndromic approach, which classifies the clinical presentation of the patient, the severity of the bite and appropriate treatment, including antivenom. This approach negates the need to identify the species of snake, focuses on managing the presenting signs and symptoms, and is very effective for early and appropriate management of snakebites. Despite the high number of patients who were admitted to the EMU, most did not have severe complications (81%). However, 46 (19%) developed severe complications that required intensive monitoring and treatment. There was no mortality during the study period using these guidelines.
Most patients who develop severe complications need some form of active medical intervention. The most common complication from snakebites was haemotoxicity (59%), usually involving a thrombocytopenia which, when significant (defined as platelets <50×109/l), required antivenom administration. The mechanism of acute thrombocytopenia is not fully understood, but antivenom reverses the effect rapidly and is preferred to platelet replacement. Rapid progressive swelling (RPS) was seen in 39% of patients with complications. The incidence of clinical compartment syndrome in snakebites is low (9% in the selected patient population with RPS), probably as most snake fangs penetrate the subcutaneous tissue rather than the muscle fascial compartment. However, RPS should be monitored closely as it can potentially cause significant morbidity. One of our patients required a fasciotomy and 3 needed limb amputations.
The use of antibiotics in RPS with blistering/bullae is not recommended in the acute setting. The snake's mouth and bite have very few micro-organisms, and venom has antibacterial properties.1 When infection occurs, the organisms responsible are usually Gram-negative enterobacteriaceae. Gas gangrene and tetanus have not been described in southern African snakebite research.
Children appear to be most vulnerable to snakebites. In our study, the highest incidence of snakebite was in the <20 years age group. Children's natural curiosity and the fact that young boys are used as herders in rural farming areas have been cited as a further risk for snakebites.6 There is also evidence that children are at higher risk for severe symptoms such as swelling, compartment syndrome and neurotoxic respiratory compromise; Blaylock suggests the high venom/body mass ratio as one of the main causes for this,1 which implies that the dose of venom in children is relatively larger than adults, making them more susceptible to life-threatening swelling or neurotoxicity. In our study, children <10 years required antivenom more than any other age group. Furthermore, children appear to be at higher risk for allergic reactions and anaphylaxis from the antivenom, as confirmed by our results with the highest incidence (60%) of antivenom-associated complications occurring in children <11 years old. The reasons for this are unclear, but a contributory factor may be that the same dose is given to adults as children.
Antivenom administration is the cornerstone of treating severe life- or limb-threatening snakebites. The dose administered is related to the type of snakebite and the amount of venom injected, and not the weight of the patient. This means that the dose should not be reduced in children. Antivenom was administered to 22 patients (9%), the most common indication being RPS (47%). The mean time to antivenom administration from the time of the bite was 16 hours. The initial dose used in this study was 40 ml polyvalent antivenom, with further doses required when patient response was inadequate (e.g. progression of swelling) and when no allergic or anaphylactic reactions were observed. Neurotoxic bites generally require higher doses of antivenom (up to 100 ml). The ideal method of antivenom administration is not clearly established, but guidelines are set in the SAIMR package insert. The effectiveness of antihistamines, hydrocortisone and adrenaline in preventing anaphylaxis prior to antivenom administration has little evidence other than a theoretical basis. The risk of acute allergic reactions and anaphylaxis is not dose-related. Furthermore, the rate of antivenom administration over 10 minutes is recommended by the manufacturer, with no evidence that a slower rate of administration is safer.1,5
The relatively high risk of allergic and anaphylactic reactions to polyvalent antivenoms has led to recommendations that it be administered in a hospital setting and not 'in the field'. Some First-World countries have developed more purified antivenoms such as the antivenin (Crotalidae) polyvalent (ACP) and the Crotalidae polyvalent immune fab (CroFab; FabAV) which are effective, with reduced adverse reactions.7,9 The decision to use antivenom should not be taken lightly, and clinicians must weigh up risks versus benefits. An understanding of the characteristics of both the venom and the antivenom is paramount. Snake venom can cause reversible (e.g. coagulopathies, such as thrombocytopenia) and irreversible (e.g. severe swelling with necrosis and tissue death) injuries. Antivenom used timeously can prevent the progression of injuries from becoming severe or lifethreatening, and can be life-saving in cases of neurotoxicity in acute settings. On the other hand are the potential adverse effects of the antivenom. In all cases where antivenom is to be administered, the attending clinician should have full resuscitation equipment and drugs on hand to manage anaphylaxis.
References
1. Blaylock R. Epidemiology of snakebite in Eshowe, Kwazulu-Natal, South Africa. Toxicon 2004; 43: 159-166.2. [ Links ]
2. Coetzer PWW, Tilbury CR. The epidemiology of snakebite in northern Natal. S Afr Med J 1981; 62: 206-212.3. [ Links ]
3. Wilkinson D. Retrospective analysis of snakebite at a rural hospital in Zululand. S Afr Med J 1994; 84: 844.4. [ Links ]
4. Marais, J. A Complete Guide to the Snakes of Southern Africa. Cape Town: Struik Publishers, 2004: 36-47.5. [ Links ]
5. South African Vaccine Producers (Pty) Ltd. SAIMR polyvalent snake antiserum/antivenom package insert. January 2006. [ Links ]
6. Hadley GP, Mars M. Snakebites in children in Africa: a practical approach to management. Surgery in Africa Monthly Reviews. University of Toronto, August 2006. [ Links ]
7. Dart RC, McNally J. Efficacy, safety and use of snake antivenoms in the United States. Ann Emerg Med 2001; 37: 181-88. [ Links ]
8. Blaylock R. The identification and syndromic management of snakebite in South Africa. South African Family Practice 2005; 47(9): 48-53. [ Links ]
9. Gold BS, Barish RA, Dart RC. North American snake envenomation: diagnosis, treatment and management. Emerg Med Clin North Am 2004; 22: 423-443. [ Links ]
Accepted 24 April 2009.
Corresponding author: D Wood (darryl.wood@kznhealth.gov.za)














