Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
SAMJ: South African Medical Journal
versión On-line ISSN 2078-5135
versión impresa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.99 no.9 Pretoria sep. 2009
ORGINAL ARTICLES
Potential for medical error: incorrectly completed request forms for thyroid function tests limit pathologists' advice to clinicians
Annalise E ZemlinI,*; Louise NuttII; Lesley J BurgessIII; Fredeline EimanIV; Rajiv T ErasmusV
IMB ChB, FCPath (Chem) SA, MMed (Chem Path), Division of Chemical Pathology, National Health Laboratory Service (NHLS), Tygerberg Hospital, Stellenbosch University, Tygerberg, W Cape. MB ChB, FCPath (Chem) SA, MMed (Chem Path), Division of Chemical Pathology, National Health Laboratory Service (NHLS), Green Point Laboratories, Green Point, Cape Town
IIMB ChB, Division of Chemical Pathology, National Health Laboratory Service (NHLS), Tygerberg Hospital, Stellenbosch University, Tygerberg, W Cape
IIIMB BS, FMCPath, FACB, DABCC, DHSM, Division of Chemical Pathology, National Health Laboratory Service (NHLS), Tygerberg Hospital, Stellenbosch University, Tygerberg, W Cape
IVMB ChB, MMed (Chem Path), PhD, Dipl International Research Ethics, TREAD Research CC, Department of Internal Medicine, Tygerberg Hospital, Stellenbosch University, Tygerberg, W Cape
VNat Dip Med Tech (Clin Path), Division of Chemical Pathology, National Health Laboratory Service (NHLS), Green Point Laboratories, Green Point, Cape Town
ABSTRACT
BACKGROUND: Various publications have highlighted the significance of laboratory errors in the pre- and post-analytical phases and their impact on results. Thyroid-stimulating hormone (TSH) is a first-line thyroid function test and, if abnormal, reflex thyroxine (T4) or tri-iodothyronine (T3) testing is requested, depending on clinical and medication data provided. Interpretative comments are added to all TFT results.
OBJECTVES: In view of the paucity of articles describing such errors, we audited laboratory request forms requesting thyroid function tests (TFT), received from primary care clinics and regional hospitals at our laboratory.
DESIGN: We assessed 482 laboratory request forms for TFT from primary health care clinics for specific parameters.
RESULTS: A total of 482 forms were analysed. Medication/s used by the patient (74.5%) and doctor's contact number (65.1%) were the most commonly incomplete parameters. Of the 123 patients with medication details, 62 (50.4%) were on thyroxine.
CONCLUSION: There are few studies examining the frequency and impact of incomplete laboratory forms on laboratory errors, and even fewer studies examining interpretative comments accompanying clinical biochemistry results. We studied how pre-analytical errors in completing request forms may lead to incorrect interpretative comments and inappropriate reflex testing, and so influence the quality of the post-analytical phase.
Laboratory quality has been historically determined by the accuracy of the analytical phase. Following the development of high-quality analytical techniques, analytical error is no longer the main reason for error in the laboratory testing process.1 Up to 68.2% of laboratory errors occur in the pre-analytical phase,2 which refers to procedures performed neither in the clinical laboratory nor under the control of laboratory personnel,3,4 e.g. completion of a laboratory request form, specimen identification, phlebotomy, sample handling and transportation to the laboratory. Post-analytical error refers to the ultimate check on the pre-and intra-analytical quality, including the reviewing pathologist providing interpretative comments, and the clinicians' interpretation and reaction to the results.3,4 Interest is growing in the assessment of clinical laboratories' contribution to medical outcomes, including the evaluation of pre-and post-analytical errors. 5
Clinical authorisation of results provides a final quality check of the entire pre-analytical and laboratory process, and is an important addition to standard quality control procedures.6 Clinical validation of biochemistry results includes the post-analytical addition of comments to a laboratory report; this should be done by a qualified person with knowledge of the potential pre-analytical and analytical variables that may influence the result.7 Providing interpretative comments, especially to primary care physicians, is an important duty of chemical pathologists.8 Owing to the paucity of clinical biochemistry training in undergraduate medical training programmes9,10 and increased specialisation of medical staff, interpretative comments may be useful for requesting physicians.6 The Royal College of Pathologists has guidelines for the provision of such comments.11 The Clinical Pathology Accreditation (CPA) Standards state that interpretation of results is an important component of clinical laboratories' services.12 External quality assessment of interpretative comments is in place in the UK, Australia and Italy.8,13-17 Clinical diagnoses are often confirmed with the use of laboratory results and, therefore, laboratory errors may lead to increased costs and unnecessary deaths. 18
According to Price, 'any test will be beneficial only if appropriate action is taken on the results'.19 Laboratory errors are important because laboratory data influence 70% of medical diagnoses and can significantly influence the success and cost of patient treatment.20 These findings have led to agreement on the definition of a laboratory error as a defect occurring at any stage of the laboratory cycle,21 and this definition has been incorporated in ISO Technical Report 22367. 22
It was previously thought that interpretative comments had little influence on patient outcome, but a study of numerous thyroid function test (TFT) requests on patients taking thyroxine replacement therapy showed that introducing interpretative comments resulted in a significant decrease in thyroxine under-replacement.23 A survey of general and nurse practitioners showed that, although interpretative comments of certain biochemical tests, including TFTs, are time-consuming, they are appreciated.24 Although interpretative comments may be useful for primary care physicians who may not be familiar with the interpretation of test results, the clinical information provided on the request forms may be limited or inappropriate and may influence the interpretative comment provided.8 Errors in interpretative comments may be attributed to the absence of adequate clinical information on request forms, which may result in comments that are misleading or harmful to patients. 25
We reviewed the compliance of laboratory request forms for TFTs received in the chemical pathology laboratory at a primary health care laboratory where interpretative comments are provided for all TFT results. We hypothesised that pre-analytical errors would influence interpretative comments and have a further impact on requests for reflex testing.
Design
Ours was a retrospective collaborative study by the Division of Chemical Pathology, NHLS, Tygerberg Hospital, and the Chemical Pathology Laboratory at Green Point Laboratories. Green Point Laboratories receive all requests from primary health care clinics in the Cape Town Metropole and surrounding areas of the Western Cape.
Original laboratory request forms received at the Chemical Pathology division during a 4-day period from 27 - 30 July 2007 requesting TFTs were manually analysed for the presence of parameters provided in Table I. These dates were chosen as they account for all TFT requests between two of the pathologist's visits. Thyroid-stimulating hormone (TSH) is a first-line thyroid function test at this laboratory and is usually the only test requested. The requesting clinician may request a thyroxine (T4) or tri-iodothyronine (T3) test, depending on the clinical situation. All TFTs at this laboratory are validated by a chemical pathologist and released with interpretative comments.
Data were captured on Excel worksheets and patient confidentiality was maintained — patients were identified by a study number only. Data analysis was by basic statistics using the Microsoft Excel programme.
The study was approved by the Ethics Committee of the University of Stellenbosch and performed according to the Declaration of Helsinki (2000).
Results
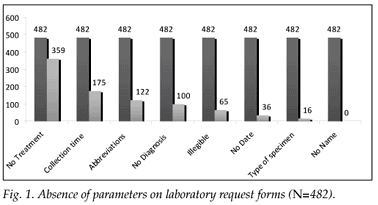
During the study period, 482 request forms for TFTs were received. Fig. 1 shows the results of the request forms when analysed for the pre-analytical quality indicators indicated in Table I. The worst parameter completed by requesting clinicians was that of medication details; 359 (74.5%) of the forms lacked this parameter; 349 (65.2%) had no contact details for the clinician; 100 (20.8%) had no diagnosis, and 122 (25.3%) had a diagnosis but in an abbreviated form. Patient and clinic details were relatively well filled in, but this might have been due to most forms being pre-stamped with clinic details, and patient identification stickers are often used. The type of specimen collected was not stated on 16 (3.3%) of forms; 36 (7.5%) did not state the date and 175 (36.3%) did not state the time of collection.
Conclusions
We have previously demonstrated that laboratory forms are not adequately completed by clinicians, and we have illustrated the impact on the communication of critical results in a tertiary care setting.26 This study shows that laboratory request forms received from primary care clinics and regional hospitals are also inadequately completed. Interpretative comments are routinely provided with all TFTs. The current use of thyroxine replacement therapy may influence these comments and should therefore be indicated on all forms. For example, a patient with a raised TSH and normal T4 may be reported as a case of subclinical hypothyroidism, when the cause may in fact be non-compliance or inadequate dosage if the patient were using thyroxine replacement therapy.
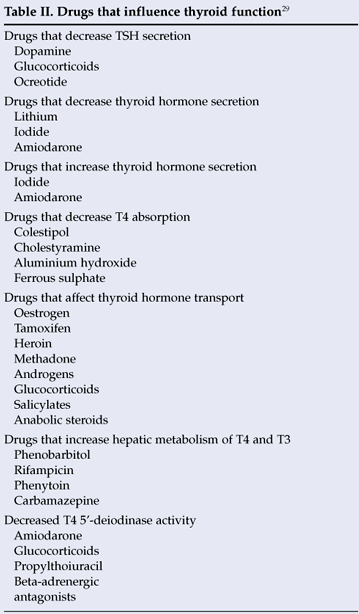
TSH is a first-line thyroid function test requested by clinicians at primary care clinics and regional hospitals. Our laboratory has its own protocol for subsequent reflex testing; if the TSH is abnormal, a reflex T4 and/or T3 is requested. Studies have found that this reflex testing permits clinicians to obtain a correct diagnosis faster and at less cost.4 A T4 is requested if the TSH is elevated in a patient not on thyroxine replacement therapy to differentiate subclinical from overt hypothyroidism. If the TSH is suppressed, a T4 is requested to differentiate subclinical from overt hyperthyroidism. If the patient is on thyroxine replacement therapy, these abnormal TSH values may indicate inadequate dosage/non-compliance or overdosage respectively. A slightly decreased TSH may also be found in nonthyroidal illness and secondary hypothyroidism, and the clinical data may hint at the probable diagnosis. Knowledge of patients' clinical and medication details is therefore important, as we do not request a T3 in patients on thyroxine replacement therapy. If this is not provided, unnecessary reflex testing of T3 is requested, resulting in an increased turnaround time and unnecessary cost. Unnecessary repeat tests in a laboratory can comprise up to 16.8% of the total workload in an immunology hospital.27 Knowledge of all medication details of patients, and not only thyroxine replacement, is also important, as many drugs can affect the interpretation of TFT results, and some may even interfere with assays (Table II). 28,29
We demonstrated that incomplete laboratory request forms may lead to misinterpretation of results, incorrect reflex test requests and inappropriate interpretative comments. Although this study was limited to TFT request forms within a primary care setting, we previously showed similar results n an academic environment.26 We only examined patients on thyroxine replacement therapy, but it would also have been of interest to examine patients on antithyroid treatment and other drugs influencing thyroid functions. 29
A limitation of this study is that the impact of incorrectly completed request forms on interpretative comments and reflex testing has not been quantified. This is difficult because of requests from numerous small outlying primary care clinics as well as regional hospitals. A follow-up on how these incorrectly completed request forms affect our services is desirable, but would be difficult to implement in our setting. Education of referring clinicians is required but would also be difficult in our setting as many of them are completing their community service in outlying primary care clinics and hospitals, resulting in lack of continuity in patient care.
In conclusion, quality assurance in the clinical laboratory is multifaceted and requires the detection of poor performance in the actions of each process. Errors in the analytical phase have been well-defined and can be compared with a gold standard, whereas errors in extra-analytical phases may be more difficult to study.30 Pre-analytical errors (e.g. absence of important clinical data on request forms) can have a serious effect on patient care by causing post-analytical errors such as inappropriate interpretative comments, as shown in this study.
We thank Dr M Rensburg for her help with the Excel charts.
References
1. Bonini P, Plebani M, Ceriotti F, Rubboli F. Errors in laboratory medicine. Clin Chem 2002; 48(5): 691-698. [ Links ]
2. Plebani M, Carraro P. Mistakes in a stat laboratory: types and frequency. Clin Chem 1997; 43(8): 1348-1351. [ Links ]
3. Plebani M. Errors in clinical laboratories or errors in laboratory medicine? Clin Chem Lab Med 2006; 44(6): 750-759. [ Links ]
4. Laposata M, Dighe A. "Pre-pre" and "post-post" analytical error: high-incidence patient safety hazards involving the clinical laboratory. Clin Chem Lab Med 2007; 45(6): 712-719. [ Links ]
5. Plebani M. Appropriateness in programs for continuous quality improvement in clinical laboratories. Clin Chim Acta 2003; 333: 131-139. [ Links ]
6. Prinsloo PJJ, Gray TA. A survey of laboratory practice in the clinical authorization and reporting of results. Ann Clin Biochem 2003; 40: 149-155. [ Links ]
7. Kilpatrick ES, Barth JH. Whither clinical validation? (editorial) Ann Clin Biochem 2006; 43: 171-172. [ Links ]
8. Marshall WJ, Challand GS. Provision of interpretative comments on biochemical report forms. Ann Clin Biochem 2000; 37: 758-763. [ Links ]
9. Freedman DB. Is the medical undergraduate curriculum 'fit for purpose'? (editorial) Ann Clin Biochem 2008; 45: 1-2. [ Links ]
10. Khromova V, Gray TA. Learning needs in clinical biochemistry for doctors in foundation years. Ann Clin Biochem 2008; 45: 33-38. [ Links ]
11. Vasik S. Interpretative commenting. Clin Biochem Rev 2008; 29 Suppl I: S99-S103. [ Links ]
12. Clinical Pathology Accreditation (UK). Standards for the medical laboratory . Sheffield: CPA (UK). http://www.cpa_uk.co.uk/files/pdlabst.pdf (accessed 22 April 2008). [ Links ]
13. Vasikaran SD, Penberthy L, Gill J, Scott S, Sikaris KA. Review of a pilot quality-assessment program for interpretative comments. Ann Clin Biochem 2002; 39: 250-260. [ Links ]
14. Challand GS, Vasikaran SD. The assessment of interpretation in clinical biochemistry: a personal view. Ann Clin Biochem 2007; 44: 101-105. [ Links ]
15. Sciacovelli L, Zardo L, Secchiero S, Zaninotto M, Plebani M. Interpretative comments and reference ranges in EQA programs as a tool for improving laboratory appropriateness and effectiveness. Clin Chim Acta 2003; 333: 209-219. [ Links ]
16. Lim EM, Sikaris KA, Gill J, Calleja J, Hickman PE, Beilby J, Vasikaran SD. Quality assessment of interpretative commenting in clinical chemistry. Clin Chem 2004; 50(3): 632-637. [ Links ]
17. Sciacovelli L, Secchiero S, Zardo L, Zaninotto M, Plebani M. External quality assessment: an effective tool for clinical governance in laboratory medicine. Clin Chem Lab Med 2006; 44(6): 740-749. [ Links ]
18. Laposata M. Patient-specific narrative interpretations of complex clinical laboratory evaluations: who is competent to provide them? (editorial) Clin Chem 2004; 50(3): 471-472. [ Links ]
19. Price CP. Evidence-based laboratory medicine: supporting decision-making. Clin Chem 2000; 46: 1041-1050. [ Links ]
20. Plebani M. Towards quality specifications in extra-analytical phases of laboratory activity (Editorial). Clin Chem Lab Med 2004; 42(6): 576-577. [ Links ]
21. Carraro P, Plebani M. Errors in a stat laboratory: types and frequencies 10 years later. Clin Chem 2007; 53(7): 1338-1342. [ Links ]
22. International Organization for Standardization. ISO/PDTS 22367. Medical laboratories: reducing error through risk management and continual improvement: complementary element . Geneva: ISO, 2005: 9. [ Links ]
23. Kilpatrick ES. Can the addition of interpretative comments to laboratory reports influence outcome? An example involving patients taking thyroxine. Ann Clin Biochem 2004; 41: 227-229. [ Links ]
24. Barlow IM. Are biochemistry interpretative comments helpful? Results of a general practitioner and nurse practitioner survey. Ann Clin Biochem 2008; 45: 88-90. [ Links ]
25. Young IS. Interpretative comments on clinical biochemistry reports. (editorial) J Clin Pathol 2005; 58: 575. [ Links ]
26. Nutt L, Zemlin AE, Erasmus RT. Incomplete laboratory request forms: the extent and impact on critical results at a tertiary hospital in South Africa. Ann Clin Biochem 2008; 45(Pt 5): 463-466. [ Links ]
27. Kwok J, Jones B. Unnecessary repeat requesting of tests: an audit in a government hospital immunology laboratory. J Clin Path 2005;58: 457-462. [ Links ]
28. Young DS, Thomas DW, Friedman RB, Pestaner LC. Effects of drugs on clinical laboratory tests. Clin Chem 1972; 18: 1041-1303 [ Links ]
29. Surks ML, Sievert R. Drugs and thyroid function. N Engl J Med 1995; 333(25): 1688-1694. [ Links ]
30. Ricós C, García-Victoria M, de la Fuente B. Quality indicators and specifications for the extra-analytical phases in clinical laboratory management. Clin Chem Lab Med 2004; 42(6): 578-582. [ Links ]
Accepted 1 April 2009.
* Corresponding author: A Zemlin (azemlin@sun.ac.za)














