Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
SAMJ: South African Medical Journal
versión On-line ISSN 2078-5135
versión impresa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.99 no.8 Pretoria ago. 2009
ORIGINAL ARTICLES
Neurocysticercosis in patients presenting with epilepsy at St Elizabeth's Hospital, Lusikisiki
Gilberto Serrano OcanaI; Juan Carlos Ortiz SablonII; Ilen Ochoa TamayoIII; Leonardo Almaguer ArenaIV; Luz Maria Serrano OcanaV; Sharlene GovenderVI, *
ISpecialist (Internal Medicine), Santiago, Cuba; MSc Infectious Diseases, Havana, Cuba, St Elizabeth's Hospital, Lusikisiki, E Cape, and Internal Medicine Department, Dora Nginza Hospital, Port Elizabeth, E Cape
IISpecialist (Internal Medicine), Santiago; MSc Critical Medicine, Havana, Internal Medicine Department, Dora Nginza Hospital, Port Elizabeth
IIISpecialist (Family Medicine), Santiago; MSc Critical Medicine, Havana, Family Medicine Department, Dora Nginza Hospital, Port Elizabeth
IVSpecialist (Family Medicine), Santiago, Lucia Inigues Hospital, Holguin, Cuba
VSpecialist (Family Medicine), Santiago, Family Medicine Department, Alcides Pino Polyclinic, Holguin
VIMSc (Microbiology), Department of Biochemistry and Microbiology, Nelson Mandela Metropolitan University, Port Elizabeth
ABSTRACT
OBJECTIVE: To survey the prevalence of neurocysticercosis in patients treated for epilepsy in Lusikisiki, E Cape.
DESIGN: This was a descriptive study. Variables considered were age, gender, symptoms and type of seizure, serological data, electroencephalogram and computed tomography (CT) findings, treatment, and ownership of pigs. Prevalence and risk assessment were determined by statistical analysis.
SUBJECTS AND SETTING: 113 patients presenting with epilepsy at St Elizabeth's Hospital, Lusikisiki, E Cape.
OUTCOME MEASURES: Prevalence of neurocysticercosis in patients presenting with epilepsy.
RESULTS: CT scans indicated that 61.1% of the patients had neurocysticercosis-associated epilepsy, the prevalence being highest in the 10 - 19-year-old age group (12.4% of the total). Neuro-imaging studies showed that calcified lesions were frequent, while active lesions were often associated with positive serological results. Non-commercial pig farming was not a significant risk factor for neurocysticercosis in the sample studied.
CONCLUSION: Neurocysticercosis was common in patients presenting with and undergoing treatment for epilepsy.
Cysticercosis is a parasitic disease caused by Taenia solium. Both people and pigs can become infected with the larvae or cysts of the parasite by faecal-oral contamination. Cysts are often located in the central nervous system (CNS), causing neurocysticercosis (NCC). Although some patients are asymptomatic, manifestations more commonly range from mild headaches to seizures, which are the main clinical feature of NCC.1 Death can occur, and it is estimated that 50 million NCC infections and 50 000 NCC-related deaths occur annually worldwide.2-4
NCC is rare in Eastern and Central Europe, North America (with the exception of Mexico), Australia, Japan, New Zealand, Israel and the Muslim countries of Africa and Asia. In Latin America and other countries in Asia and Africa, NCC is endemic and poses a serious public health problem.5-7 A high prevalence has been reported in immigrant populations in the southwestern USA and South Africa.8
NCC may present with a variety of clinical manifestations, so complementary studies are required for confirmation of the diagnosis.7 The parenchymatous cysts are usually small (approximately 10 mm), single or multiple and found mainly in the cerebral cortex and basal ganglia.6 The parenchymal form is most common and accounts for 29 - 62% of cases of NCC,7 causing headache, seizures, focal neurological deficit or intellectual impairment.4-7,9,10 The subarachnoid form is responsible for 27 - 56% of cases.6 Symptoms of intraventricular cysticercosis include obstructive hydrocephalus,3 and extraneural cysticercosis may occur in the eyes,11 muscles and subcutaneous tissue. Presence of cysts in the subarachnoid space is rare.7 Seventy-five per cent of patients with NCC have calcified cysts in the muscles.7-9
The diagnosis of NCC is difficult because clinical manifestations are nonspecific, most neuro-imaging findings are not pathognomic, and some serological tests have low sensitivity and specificity.12 A set of diagnostic criteria has been proposed based on objective clinical, imaging, immunological and epidemiological data.13 These criteria consist of four categories that are stratified according to their diagnostic strength.12 Clinically it may be difficult to differentiate between cysticercosis and other parasitic diseases. Nevertheless, epidemiological data, evidence provided by neuro-imaging studies and serological tests usually provide useful diagnostic clues.12
Treatment of NCC includes antiparasitic drugs (praziquantel 50 mg/kg/d for 15 days and albendazole 15 mg/kg/d for 8 - 15 days),14-17 surgery, and symptomatic and anti-inflammatory medication12 (corticosteroids, e.g. dexamethasone 16 - 32 mg/d).17
Our study setting was the small town of Lusikisiki in the Qaukeni health district, Eastern Cape. Lusikisiki has a population of 150 000 and is served by a regional hospital (St Elizabeth's Hospital) and 12 local clinics. We aimed to: (i) compare serological and computed tomography (CT) findings for the diagnosis of NCC; (ii) determine whether age and gender were associated with being seropositive for NCC; and (iii) assess whether non-commercial pig farming was a risk factor for NCC.
Methods
One hundred and thirteen consenting patients presenting with epilepsy at St Elizabeth's Hospital, Lusikisiki, in 2004 participated in the study. Ethical approval was obtained from the Research Ethics Committee, Walter Sisulu University, Mthatha (reference number 0018-05).
This was a prospective study in which known epileptic patients were randomly selected and sent to Nelson Mandela Academic Hospital, Mthatha, for brain CT scans (during the study period the hospital could only accommodate a maximum of 113 patients for CT scans). Skull radiographs (anteroposterior and lateral views) and the results of serological tests performed by the National Health Laboratory Service, Johannesburg, were also analysed.
The diagnosis of epilepsy was made clinically. All the patients in the study had presented with two or more unprovoked seizures. According to the hospital records, these were generalised tonic-clonic (54 cases), secondary generalised (26), focal motor (31) and temporal lobe (2) seizures. The following variables were considered: age, gender, symptoms, type of seizure, treatment, serological results, pig ownership, and electroencephalogram and CT scan findings. The lesions seen on CT scans were classified as set out in Table I.
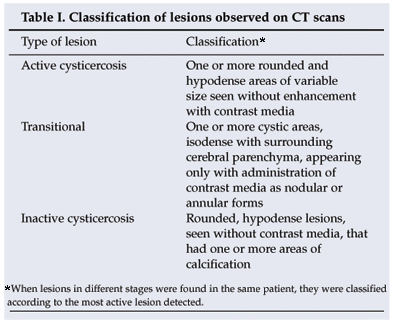
Statistical analysis of the data was performed using the Epi Info version 3.5.1 software program, and recommendations for prevention and diagnosis were issued to patients and hospital staff.
Results
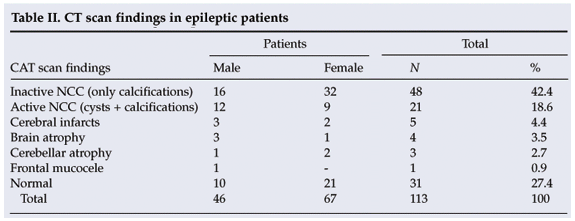
CT scans indicated that 61.1% (69/113) of the patients had NCC (inactive and active). There was no significant association (p>0.05) between gender and NCC (Table II). Serological tests for cysticercosis (IgG) were positive in 37.2% of patients (42/113), negative in 44.2% (50/113) and inconclusive in 18.6% (21/113). There was no significant difference between males and females with regard to the seroprevalence of NCC.
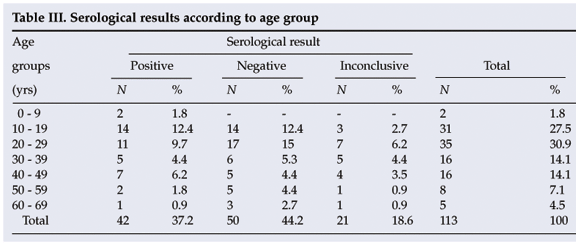
In this study the seroprevalence of cysticercosis was found to be highest in the age group 10 - 19 years (12.4% of the total), followed by 20 - 29 years (9.7%) and 40 - 49 years (6.2%). No significant differences were found (p>0.05). The average age of the patients in this study was 29.3 years, with the youngest being 5 and the oldest 67 (Table III).
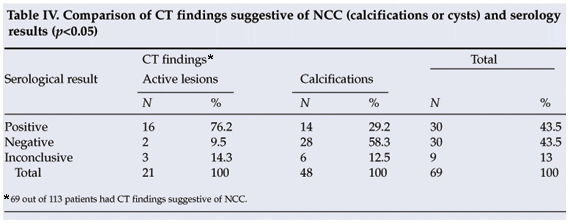
In 16 out of 21 patients with active NCC lesions serological studies were positive for NCC, in 2 they were negative, and in 3 cases results were inconclusive (Table IV). There was a significant (p<0.05) correlation between the presence of active lesions on CT and positive serological findings. Of the 48 patients in whom CT scans showed calcifications, 14 (29.2%) were serologically positive for NCC and 28 (58.3%) negative, while in 6 cases (12.5%) the results were inconclusive. Positive serological results were obtained in 12 patients who had no radiological evidence of NCC (10 had a normal CT scan and in 2 the scan showed brain atrophy).
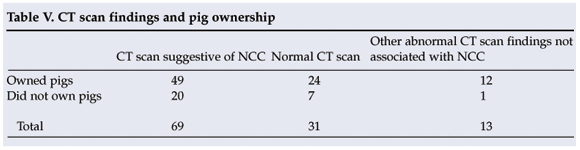
There was no significant difference (p>0.05) between the patients who owned pigs and had a CT scan suggestive of NCC and those who owned pigs and had a normal CT scan (Table V). Pig ownership was therefore not shown to be a risk factor (attributable risk 0.471) for NCC in the sample group studied at Lusikisiki.
Discussion
CT scan findings in this study correlated with the characteristic NCC neuro-imaging features (appearance of scolex as a hyperintense nodule, punctuate calcifications) reported in other studies.6,10,11,14,15,18 It has been documented that magnetic resonance imaging (MRI) is preferable to CT scanning for the diagnosis of NCC, particularly in patients with skull-base lesions, brainstem cysts, intraventricular cysts and spinal lesions. However, inaccuracies in MRI detection of small calcifications have been reported.6,10,11,15,19
In this sample group, 61% of patients with epilepsy (2 or more seizures) were found to have NCC, which is a higher figure than those reported in India (active lesions of NCC in 34.6% of patients with multiple seizures and 59.2% of those with a single seizure)20 and Madagascar (NCC in 12.6% of patients with epilepsy).21
Serological studies may provide information that can assist in the diagnosis of cysticercosis, but should be used in conjunction with clinical, epidemiological and radiological criteria. Although enzyme-linked immuno-electrotransfer blot assay (EITB) is recommended for NCC immunodiagnosis,18 enzyme-linked immunosorbent assay (ELISA) is more commonly used for epidemiological studies because it is technically simpler and cheaper.22 False-positive ELISA results have however been reported in patients with other helminth infections such as Echinococcus.6,23 Discrepancies between EITB and CT findings have also been documented, where serological tests were negative in over 50% of NCC patients with positive CT scans.24
A positive serological test for cysticercosis may simply indicate that the patient has had contact with T. solium antigens in the form of eggs, cysts or adult parasites. Cysticercosis can also be located in another organ, such as skeletal muscle, or may already be resolved with circulating antibodies. Immunological tests are more specific when cerebrospinal fluid (CSF) is analysed (sensitivity of 87%, specificity 95%).6,7,23 The high seroprevalence found in this study may be due to patient exposure to T. solium antigens (eggs, cysts or adult parasite) and unsanitary environmental conditions. The seroprevalence of NCC in patients with epilepsy in hospital settings has been variously reported as 26% in India, 12% in Peru, and 29% in Mexico.25 Seroprevalence rates in rural Mexico, Guatemala, Honduras, Ecuador and Bolivia ranged from 10% to 23%.26
We acknowledge that the sample size in our study was too small for any generalised conclusions to be drawn. The low number of patients investigated with CT scans was due to limited economic and financial resources in the study area, as the nearest CT scanner was more than 130 km away in a hospital serving >1.5 million people. Another limitation of this study was financial constraints and the lack of an MRI facility for patient examination.
Although there was no statistically significant difference (p>0.045) between the patients who owned pigs and had CT scans suggestive of NCC and those who owned pigs and had normal CT scans, it should be noted that the generally unsanitary living conditions in the study setting of Lusikisiki may have contributed to the high prevalence of NCC in epileptic patients.
Cysticercosis is a preventable disease that is responsible for a significant number of cases of epilepsy in most developing countries.26 It can only be controlled and eradicated by improving living and sanitary conditions in areas in which it is endemic. The possibility of NCC should always be considered by physicians evaluating patients with epilepsy who live in or come from endemic zones.27,28
Recommendations include educating the community on the importance of maintaining proper sanitary conditions to reduce the incidence and prevalence of taeniosis/cysticercosis. Furthermore, all patients with seizures of recent onset should have a CT or MRI scan of the brain. In poor areas with limited health facilities and economic resources, serological tests for cysticercosis should always be considered. To exclude NCC, CT or MRI should be requested if a patient has a positive serological result.
References
1. Lescano AG, Garcia HH, Gilman RH, et al. Taenia solium cysticercosis hotspots surrounding tapeworm carriers: clustering on human seroprevalence but not on seizures. PLOS Neglected Trop Dis 2009; 3: e371 doi:10.1371/journal.pntd.0000371. [ Links ]
2. Alarcón F. Neurocysticercosis: its aetiopathogenesis, clinical manifestations, diagnosis and treatment. Rev Neurol 2006; 43: S93-00. [ Links ]
3. Kalra S, Jaiswal AK, Behari S, Jain VK. Lateral ventricular neurocysticercosis: A case report. Indian J Radiol Imaging 2006; 16: 775-778. [ Links ]
4. Psarras TG, Zour A, Coimbra C. Neurocysticercosis: A neurosurgical perspective. South Med J 2003; 96 (10): 1019-1022. [ Links ]
5. Del Brutto OH. Neurocysticercosis. Semin Neurol 2005; 25 (3): 243-251. [ Links ]
6. Sotelo J, Del Brutto OH. Review of neurocysticercosis. Neurosurg Focus 2002; 12 (6). [ Links ]
7. Kraft R. Cysticercosis: An emerging parasitic disease. Am Fam Physician 2007; 76(1): 91-96. [ Links ]
8. Burneo JG. Neurocysticercosis. 2006. http://emedicine.medscape.com/ article/1168656-overview (accessed 22 October 2008). [ Links ]
9. White AC. Neurocysticercosis: Updates on epidemiology, pathogenesis, diagnosis, and management. Ann Rev Med 2000; 51: 187-206. [ Links ]
10. Antoniuk S, Bruck I, Santos LH, Souza LP, Fugimura S, Neurocysticercosis in children: clinical study and follow-up of 112 patients. Rev Neurol 2006; 42: S97-01. [ Links ]
11. Kaliaperumal S, Rao VA, Parija SC. Cysticercosis of the eye in South India – a case series. Indian J Med Microbiol 2005; 23: 227-230. [ Links ]
12. Garcia HH, Evans CAW, Nash TE, et al. Current consensus guidelines for treatment of neurocysticercosis. Clin Microbiol Rev 2002; 15: 747-756. [ Links ]
13. Del Brutto OH, Rajshekhar V, White AC, et al. Proposed diagnostic criteria for neurocysticercosis. Neurology 2001; 57: 177-183. [ Links ]
14. Rosenfeld E. Neurocysticercosis update. J Pediatr Infect Dis 2003; 22: 181-182. [ Links ]
15. Sharma, S, Agarwal A, Kar AM, Shukla R, Garg RK. Evaluation of role of steroid alone and with albendazole in patients of epilepsy with single-small enhancing computerized tomography lesions. Ann Neurol Indian Acad 2007; 10: 39-43. [ Links ]
16. Pérez-López C, Isla-Guerrero A, Álvarez F, et al. Update in neurocysticercosis treatment. Rev Neurol 2003; 36 (9): 805-811. [ Links ]
17. Carod Artal FJ. Stroke in central nervous system infections. Ann Neurol Indian Acad 2008; 11: 64-78. [ Links ]
18. Kojica EM, White CA. A positive enzyme-linked immuno-electrotransfer blot assay result for a patient without evidence of cysticercosis. Clin Infect Dis 2003; 36: e7–e9. [ Links ]
19. Ferreira LS, Zanardi VA, Li Min L, Guerreiro Marilisa M. Inter-relationship between radiologic findings and prognosis of epilepsy in children with neurocysticercosis. Arq Neuro-Psiquiatr 2002; 60(1): 1-5. [ Links ]
20. Singh G, Singh I, Rani A, Kaushal S, Avathi G. Epidemiologic classification of seizures associated with neurocysticercosis: observations from a sample of seizure disorders in neurologic care in India. Acta Neurol Scand 2006; 13 (4): 233-240. [ Links ]
21 Adriantseheno LM, Adrianasy TF. The features of epilepsy in Malagasy. A hospital study on 213 cases from the north western part of Madagascar. Afr J Neurol Sci 1997; 16(2): 28-33. [ Links ]
22. Carpio A. Neurocysticercosis: an update. Lancet Infect Dis 2002; 2: 751-762. [ Links ]
23. Garg RK. Diagnostic criteria for neurocysticercosis: Some modifications are needed for Indian patients. Neurol India 2004; 52: 171-177. [ Links ]
24. Goodman KA, Ballagh SA, Carpio A. Case-control study of seropositivity for cysticercosis in Cuenca, Ecuador. Am J Trop Med Hyg 1999; 60(1): 70-74. [ Links ]
25. Palacio LG, Jimmenez I, Garcia H, et al. Neurocysticercosis in persons with epilepsy in Medellin, Colombia. Epilepsia 1998; 39(12): 1334-1339. [ Links ]
26. Román G, Sotelo J, Del Brutto O, et al. A proposal to declare neurocysticercosis an international reportable disease. Bull World Health Organ 2000; 78(3): 399-406. [ Links ]
27. Bern C, Garcia HH, Evans C, et al. Magnitude of the disease burden from neurocysticercosis in a developing country. Clin Infect Dis 1999; 29: 1203-1209. [ Links ]
28. Prasad KH, Prasad A, Verma A, Singh AK. Human cysticercosis and Indian scenario: a review. J Biosci 2008; 33(4): 571-582. [ Links ]
Accepted 24 April 2009.
* Corresponding author: S Govender (sharlene.govender@nmmu.ac.za)














