Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
SAMJ: South African Medical Journal
versión On-line ISSN 2078-5135
versión impresa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.99 no.6 Pretoria jun. 2009
GUIDELINE
Venous thromboembolism – prophylactic and therapeutic practice guideline
B F Jacobson; S Louw; M Mer; S Haas; H R Büller; A T O Abdul-Carim; D Adler; A Beaton; P R de Jong; D van der Jagt; B Levy; J Pearl; E Schapkaitz; P Wessels
On behalf of the Southern African Society of Thrombosis and Haemostasis
ABSTRACT
BACKGROUND: Pharmacological prophylactic anticoagulation in many countries, including South Africa, is under-prescribed, which unfortunately results in unacceptable morbidity and mortality in a substantial number of patients.
METHOD: The Southern African Society of Thrombosis and Haemostasis reviewed the available literature as well as guidelines from other societies. Specialties represented on the committees included anaesthetics, cardiology, clinical haematology, critical care, gynaecology, haematopathology, internal medicine, neurology, orthopaedic surgery, pulmonology and vascular surgery. A draft document was produced, which was revised by consensus agreement. To avoid local bias, the guidelines were adjudicated by recognised independent international external experts.
RESULTS AND CONCLUSION: A concise, practical guideline for thrombo-prophylaxis and treatment in medical and surgical patients has been produced for South African conditions. These guidelines will hopefully lead to improved anticoagulation practice in this country, which we believe will directly benefit patient outcomes.
These guidelines for prophylactic anticoagulation reflect current best practice. However, every patient should still be assessed on merit, with individualisation of therapy where indicated. Drug recommendations are based on Medicines Control Council registration at the time of publication, unless otherwise indicated.
1. Medical patients
1.1 Background
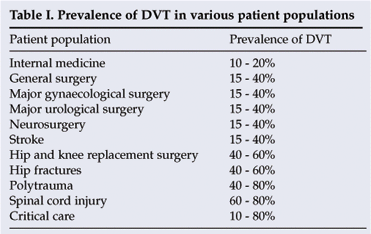
In the absence of anticoagulation, the risk of deep vein thrombosis (DVT) in medically ill patients is comparable to that observed in moderate-risk surgical patients: 10 - 20%. Although the clinical significance of asymptomatic distal DVT is debatable, pulmonary embolism (PE) is the most common preventable cause of death in hospital patients, accounting for 10% of all hospital deaths. Three-quarters of these deaths occur in medically ill patients. The efficacy of heparins in preventing venous thromboembolism (VTE) in medically ill patients is now well established. However, their use is associated with an increased risk of major bleeding episodes, and this factor should be balanced against the thrombotic risk.
1.2 Risk assessment
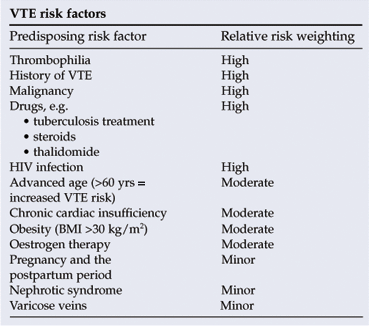
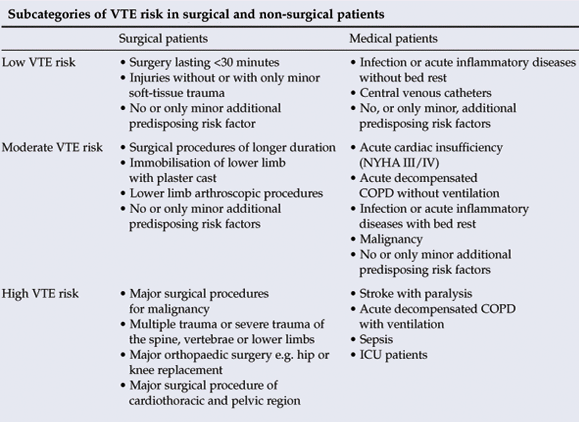
Risk assessment is essential and treatment needs to be individualised. Refer to Appendix 1 for further information.
1.3 Recommendations
1.3.1 Prophylaxis in medically ill patients
The recommended prophylactic doses for low-molecular-weight heparin (LMWH) and unfractionated heparin are as follows:
• enoxaparin 40 mg (4 000 anti-Xa units) sc (subcutaneous) daily
or
• unfractionated heparin (UFH) 5 000 units sc tds (3 times per day).
Evidence-based data show LMWH to be superior to unfractionated heparin.
LMWH should be given to all bedridden medically ill patients who have:
• conditions associated with a high risk of thrombosis (see Appendix 1)
or
• other medical conditions (such as acute infections or acute rheumatic disease) with at least one associated risk factor.
Note: Prophylaxis is not required for patients who are mobile.
In patients at high risk of bleeding, the use of mechanical prophylaxis such as graduated compression stockings (GCS) or intermittent pneumatic compression (IPC) devices should be considered as an alternative if the thrombotic risk is high.
1.4 Monitoring – see General recommendations
2. Surgical patients
2.1 Background
Refer to the comments above (under 1.1) regarding background and risk assessment. Furthermore, VTE is an important cause of morbidity and mortality in surgical patients. However, the relative risk of developing VTE varies among patients, and some measure of risk assessment is required for appropriate selection of prophylaxis. The following guidelines attempt to simplify risk assessment models, which are often too complicated for routine use.
Both patient-related and procedure-related risk factors should be considered when assessing an individual's risk of developing VTE.
2.1.1 Patient-related risk factors for VTE
• age >60
• previous history of VTE
• immobility
• underlying malignancies
• pregnancy
• oestrogen replacement therapy
• obesity
• underlying hereditary thrombophilic state
• underlying inflammatory bowel disease.
2.1.2 Procedure-related risk factors
• duration of procedure
• degree of tissue damage (orthopaedic and trauma surgery carry the greatest risk)
• degree of immobility following surgery
• nature of surgical procedure (e.g. lower limb orthopaedic surgery, neurosurgery, etc.).
2.2 Recommendations
2.2.1 Prophylaxis in surgical patients
2.2.1.1. Patients undergoing low-risk procedures (minor surgery) with no patient-related risk factors:
• no specific prophylaxis is required
• early mobilisation is recommended.
2.2.1.2. Patients undergoing higher-risk procedures (major surgery) with no patient-related risk factors OR undergoing low-risk procedures with additional patient-related risk factors:
Enoxaparin 40 mg (4 000 anti-Xa units) sc daily
or
Dalteparin 0.2 ml (2 500 anti-Xa units) sc daily.
or
Nadroparin.
Abdominal surgery: 0.3 ml (2 850 anti-Xa units) sc 2 hours pre-operatively and 8 hours after surgery, followed by 0.3 ml daily for 7 days.
Knee and hip replacement surgery: Weight-adjusted dose of 38 anti-Xa units/kg sc 12 hours preoperatively and repeated 12 hours after surgery and daily on days 1 - 3 with 57 anti-Xa units/kg sc from day 4 for a minimum of 10 days.
Prophylaxis should generally be given 12 hours before surgery and once-daily post-operatively (see Timing of prophylaxis (2.2.2) below). Intermittent pneumatic compression (IPC) devices may be acceptable alternatives, particularly if minor bleeding is likely to be harmful or if other factors suggest an increased bleeding risk. Therefore, if feasible and practical, IPC devices should be utilised.
2.2.1.3. Patients undergoing higher-risk procedures (major surgery) with additional patient-related risk factors OR undergoing very high-risk procedures (orthopaedic or trauma surgery):
Enoxaparin 40 mg (4 000 anti-Xa units) sc daily
or
Dalteparin 0.4 ml (5 000 anti-Xa units) sc daily
or
Nadroparin.
Abdominal surgery: 0.3 ml (2 850 anti-Xa units) sc 2 hours pre-operatively and 8 hours after surgery, followed by 0.3 ml daily for 7 days.
Knee and hip replacement surgery: weight-adjusted dose of 38 anti-Xa units/kg sc 12 hours pre-operatively and repeated 12 hours after surgery and daily on days 1 - 3, with 57 anti-Xa units/kg sc from day 4 for a minimum of 10 days.
Fondaparinux 2.5 mg sc daily (NB: only registered for prophylaxis in total hip and knee replacement surgery and arthroplasty, and only to be administered after surgery).
See paragraphs below on timing and duration of prophylaxis. Consideration should be given in this group of patients to using additional mechanical devices such as IPC devices.
2.2.2 Timing of prophylaxis
This is an extremely controversial matter! Data are available confirming the benefits of prophylactic anticoagulation initiated pre-operatively that, although desirable, is not always possible. However, it should be given postoperatively within 6 - 12 hours (6 hours if bleeding risk is minor and 12 hours if bleeding risk is major) i.e. not before 6 hours postoperatively and not after 12 hours postoperatively, provided that no active bleeding is present. For major hip and knee surgery, a postoperative initiation time of 12 hours appears to be optimal.
Adjustment of the dose with renal failure is mandatory (refer to package inserts of individual drugs). The first dose of fondaparinux should always be given 6 - 8 hours postoperatively. In patients at high risk of bleeding or undergoing regional anaesthesia (see separate guidelines), anticoagulation should always only be initiated 12 hours postoperatively. Not all experts agree that pre-operative dosing is essential.
2.2.3 Duration of prophylaxis
• General recommendations:
• major cancer surgery: 5 weeks
• hip replacement surgery: 5 weeks
• knee replacement surgery: 2 weeks
• LMWH prophylaxis should be continued until the patient is fully mobile.
• For major surgery in patients with additional risk factors or very high-risk procedures (e.g. major orthopaedic surgery), at least 7 - 10 days' prophylaxis is indicated.
• Extended out-of-hospital prophylaxis (up to 1 month) with LMWH or warfarin started immediately postoperatively and adjusted to maintain an international normalised ratio (INR) of 2 - 3 has been shown to provide additional benefit.
For monitoring of LMWH, see General recommendations.
2.2.4 Special circumstances
2.2.4.1 Recommendations with reference to centroneuro-axial blockade (spinal and epidural anaesthesia) in the setting of prophylactic doses
• Catheter should not be placed or removed within 12 hours after a dose of LMWH.
• LMWH should not be commenced less than 2 hours after insertion or removal of a neuro-axial catheter.
• LMWH should be delayed at least 24 hours if there is blood in the needle or neuro-axial catheter during needle insertion.
• Neurological monitoring is mandatory for a minimum of 12 hours and ideally for 72 hours after neuro-axial blockade in association with anticoagulation.
• Extreme caution should be exercised in patients on other haemostatically active agents such as aspirin and non-steroidal anti-inflammatory agents.
• Currently, only limited data are available on the utilisation of fondaparinux in this setting. The committee is therefore unable to make definitive recommendations for this drug. Because of the drug's long half-life, catheter removal should not take place less than 36 hours after cessation of fondaparinux.
2.2.4.2 Recommendations with reference to prophylaxis in pregnancy
There are no drugs registered for these indications in pregnancy; these are therefore off-label recommendations. (All other recommendations in these guidelines are as per registered indications/'on-label'.)
• In healthy pregnant women undergoing a procedure (e.g. caesarean section) under centroneuro-axial blockade or general anaesthesia with no specific risk factors, it is recommended that non-pharmacological methods and early mobilisation be practised.
• In pregnant women with risk factors for thrombosis (e.g. obesity), LMWH prophylaxis is safe for the mother and fetus and should be commenced within 6 hours after caesarean section/normal vaginal delivery as described under Prophylaxis in surgical patients (2.2.1) above. However, if neuro-axial anaesthesia has been used, initiation of prophylaxis should be delayed for 12 hours.
2.2.5 General recommendations
2.2.5.1 Monitoring of patients on LMWH
• The patient's platelet count should be checked on initiation of LMWH, after 5 days, and regularly thereafter while on therapy.
• Anticoagulant activity is measured using an anti-Xa activity assay.
• Anti-Xa measurement is only indicated in pregnancy, renal failure or excessively obese patients for whom large doses are required.
• The anti-Xa assay must be calibrated for each LMWH tested.
• The anti-Xa assay for enoxaparin and nadroparin is currently available from the Johannesburg Hospital Haematology Laboratory (011 488-3068 or 011 489-8552), as well as from most private laboratories.
• 5 ml citrated blood taken 3 hours post LMWH dose is required for the assay.
• Target levels
• Prophylaxis target is 0.3 - 0.5 anti-Xa units/ml of blood.
• Therapeutic target is 0.6 - 1.0 anti-Xa units/ml of blood.
• Target values for therapeutic anticoagulation in pregnant patients with an artificial cardiac valve is 1 - 1.2 anti-Xa units/ml of blood.
2.2.5.2 Management of bleeding patients
• Do not use prophylaxis if there is severe bleeding.
• Discontinue LMWH as well as any other haemostatically active agents that may contribute to haemorrhage.
• Supportive care includes transfusion of blood products.
• Measurement of anti-Xa levels may be indicated.
• Protamine sulphate is effective in neutralising the antithrombin activity of LMWH but has limited effect on the anti-Xa activity. Consider protamine sulphate if bleeding is severe.
• A dose of 1 mg protamine sulphate reverses the effect of 100 anti-Xa units of LMWH (1 mg enoxaparin is equivalent to ~100 anti-Xa units). To reverse the effect of 40 mg enoxaparin, 40 mg protamine sulphate is needed.
• If the patient continues to bleed, a repeat dose of 0.5 mg protamine sulphate per 100 anti-Xa units of enoxaparin is indicated, and can be repeated to a maximum of 3 doses.
3. Guidelines for treatment of venous thrombo-embolism (VTE)
3.1 Initiation of anticoagulation
LMWH offers definite advantages over UFH because not only is the dosing convenient but there is also no need to monitor patients, which allows for the possibility of outpatient management for certain patients; it may also result in a reduced risk of recurrence. The guidelines below pertain to the use of LMWH.
Enoxaparin 1 mg/kg sc twice daily
or
Nadroparin weight adjusted 0.1 ml/10 kg sc twice daily (according to manufacturer's guidelines)
or
Dalteparin 100 anti-Xa units/kg sc twice daily.
• The above drugs should be given for at least 7 days.
• Warfarin should be started at a dose of 5 mg po daily from day 2 of anticoagulation. The practice of giving a 'loading dose' has been discontinued.
• The INR should be measured 2 - 3 days after starting warfarin and then daily, with dose adjustments to achieve a therapeutic range of 2 - 3 (for most indications).
• LMWH must be given for at least 7 days, even if the INR has reached therapeutic level.
• LMWH can be discontinued once the INR has been in the therapeutic range for 2 consecutive days.
• For massive thrombosis or pulmonary embolism, LMWH should be given for 7 - 10 days.
• For massive pulmonary embolism, thrombolytic therapy is indicated in the presence of haemodynamic compromise e.g. r-TPA 100 mg IV over 2 hours.
3.2 Duration of oral anticoagulation
The duration needs to be individualised according to the patient's thrombo-embolic risk level; only basic recommendations are given here.
• Patients with reversible or time-limited risk factors should be treated for at least 3 months.
• Patients with idiopathic DVT and all patients with PE should be treated for at least 6 months.
• Patients with recurrent idiopathic VTE, or continuing risk factors, or who have had a life-threatening event, or have had a thrombosis in an unusual site, may benefit from longer duration anticoagulation, possibly lifelong in some patients.
• Underlying antithrombin deficiency, antiphospholipid syndrome or malignancy carry the highest risk of relapse, and lifelong anticoagulation as secondary prophylaxis should be considered in these settings. The presence of multiple concurrent risk factors also significantly increases the risk of recurrence.
• NB: The presence of heterozygous factor V Leiden is a weak independent risk factor for recurrence and is not an indication for long-term anticoagulation following a first event.
• Spontaneous superficial thrombophlebitis: Current data suggest intermediate dose LMWH for 1 month.
3.3 Venacaval filters
Venacaval filters are indicated in the following circumstances:
• recurrent VTE despite adequate anticoagulation
• contraindications to anticoagulation, such as head injury
• inability to achieve optimal anticoagulation.
3.4 Catheter-directed thrombolysis
This may be indicated in centres with the necessary expertise for young healthy patients with a large clot burden, especially in ilio-femoral thrombosis.
3.5 Thrombophilia screening
The presence of an underlying hereditary thrombophilic state does not alter initial management, and thrombophilia screening should be delayed until 2 weeks after discontinuation of therapy because the results are altered by the acute event and by anticoagulant therapy.
3.6 Outpatient management
Management of VTE in the outpatient setting is safe and cost-effective provided that:
• the patient is able to understand and administer therapy himself/herself
• the patient is able to attend regular follow-up and has rapid access to hospital care should it be required
• no complicating factors (e.g. increased bleeding risk) are present.
3.7 Management of non-therapeutic INRs
This should be individualised according to bleeding risk. Below are general guidelines:
• INR <5 no significant bleeding:
• omit warfarin
• re-start lower dose once INR is in the therapeutic range.
• INR >5<10 no significant bleeding:
• omit warfarin
• monitor INR daily until back in therapeutic range
• restart warfarin at lower dose
• consider low-dose oral vitamin K if INR remains prolonged.
• INR >10 no significant bleeding:
• stop warfarin
• give vitamin K 2 mg po
• monitor INR daily until in therapeutic range (repeat vitamin K as required)
• restart warfarin at lower dose.
• Patients with significant bleeding:
• stop warfarin
• give fresh frozen plasma (FFP) or bioplasma or prothrombin complex concentrate (if bleed is life-threatening)
• give vitamin K slowly intravenously.
Special precautions should be exercised when reversing anticoagulation in patients with prosthetic heart valves.
The authors thank Hilda Jacobson for editing the manuscript.
These guidelines have been endorsed by the Southern African Society of Thrombosis and Haemostasis, the Critical Care Society of South Africa, the South African Society of Anaesthesiologists, the South African Society of Obstetricians and Gynaecologists, the South African Orthopaedic Association and the South African Society of Cardiovascular Intervention.
Bibliography
Caprini JA, Arcelus JI, Reyna JJ, et al. Effective risk stratification of surgical and non-surgical patients for venous thromboembolic disease. Semin Hematol 2001; 38(2): Suppl 5, 12-19. [ Links ]
Chai SJ, Macik BG. Improving the safety profile of warfarin. Semin Hematol 2002; 39(3): 179-186. [ Links ]
Cohen A. Benefits of DVT prophylaxis in the non-surgical patient. Semin Hematol 2001; 38(2): Suppl S, 531-538. [ Links ]
Dahl OE, Bergqvist D. Current controversies in DVT prophylaxis after orthopaedic surgery. Curr Opin Pulm Med 2002; 8(5): 394-397. [ Links ]
Eight American College of Chest Physicians (ACCP) Consensus Conference on Antithrombotic Therapy. Chest 2008; 133: 1293-1295. [ Links ]
Hirsh J, Warkentin TE, Shaughnessy SG, et al. Heparin and LMWH mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy and safety. Chest 2001; 119: 64S-94S. [ Links ]
Hull RD, Pineo GF, MacIsaac S. Preoperative vs. postoperative initiation of LMWH prophylaxis against VTE in patients undergoing elective hip replacement. Arch Intern Med 1999; 159: 137-141. [ Links ]
Hyers TM, Agnelli G, Hull RD, et al. Antithrombotic therapy for venous thromboembolic disease. Chest 1998; 114: suppl 5, 561S-578S. [ Links ]
Kearon C, Gent M, Hirsh J, et al. Comparison of 3 months of anticoagulation with extended anticoagulation for a first episode of idiopathic VTE. N Engl J Med. 1999; 340(12): 901-907. Erratum in: N Engl J Med 1999; 341(4): 298. [ Links ]
Mismetti P, Laporte-Simitsidis S, Tardy B, et al. Prevention of VTE in internal medicine with UFH or LMWH - A meta analysis of randomised clinical trials. Thromb Haemost 2000; 83(1): 14-19. [ Links ]
Nicolaides AN, Fareed J, Kakkar AK, et al. Prevention of VTE: International Consensus Statement (guidelines according to scientific evidence). Int Angiol 1997; 16(1): 3-38. [ Links ]
O Shea S, Ortel TL. Issues in the utilization of LMWH. Semin Hematol 2002; 39(3): 172-178. [ Links ]
Palareti G. Bleeding complications of oral anticoagulant treatment. Lancet 1996; 348: 423-428. [ Links ]
Pottier P, Planchon B, Truchaud F, et al. Rationalisation of risk factors for venous thromboembolism in medical in-patients: a prospective study. J Mal Vasc 2000; 25(4): 241-249. [ Links ]
Prandoni P. Heparins and venous thromboembolism: Current practice and future directions. Thromb Haemost 2001; 86: 488-498. [ Links ]
Samama MM, Cohen AT, Darmon JY, et al. Comparison of enoxaparin with placebo for the prevention of VTE in acutely ill medical patients. N Engl J Med 1999; 341: 793-800. [ Links ]
Scurr J, Baglin T, Burns H, et al. Second Thromboembolic Risk Factors (THRIFT II) Consensus Group: Risk of prophylaxis for VTE in hospital patients. Phlebology 1998; 13: 87-97. [ Links ]
Walker ID, Greaves M, Preston FE. Guidelines for investigation and management of heritable thrombophilia. Br J Haematol 2001; 114(3): 512-528. [ Links ]
Correspondence to: Professor B F Jacobson, Department of Haematology, National Health Laboratory Service and University of the Witwatersrand, PO Box 1038, Johannesburg 2000, tel. 011 489 8414, e-mail: clot@nhls.ac.za
Risk assessment: VTE risk factors can be divided into predisposing factors (i.e. patient characteristics) and exposing factors (i.e. some medical conditions, nature of surgical intervention, etc.) (see tables).