Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.99 no.5 Pretoria Mai. 2009
ORIGINAL ARTICLES
Measles outbreak in South Africa, 2003 - 2005
Meredith L McMorrowI, *; Goitom GebremedhinII; Johann van den HeeverIV; Robert KezaalaV; Bernice N HarrisVI; Robin NandyVII; Peter StrebelV; Abdoulie JackIII; K Lisa CairnsVIII
IMD, MPH, Malaria Branch, Division of Parasitic Diseases, National Center for Zoonotic, Vector-Borne, and Enteric Diseases, Centers for Disease Control and Prevention and United States Public Health Service, Atlanta, USA
IIMD, MPH, World Health Organization, Abuja, Nigeria
IIIMD, PhD, World Health Organization, Abuja, Nigeria
IVBSocSc (Hons), MSocSc, Expanded Programme on Immunisation, Department of Health, Pretoria
VMB ChB, MPH, Department of Immunization, Vaccines, and Biologicals, World Health Organization, Geneva, Switzerland
VIMB ChB, MMed, National Institute for Communicable Diseases, National Health Laboratory Service, Johannesburg, and School of Health Systems and Public Health, University of Pretoria
VIIMBBS, MPH, Global Immunization Division, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, USA
VIIIMD, MPH, Global Immunization Division, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, USA
ABSTRACT
OBJECTIVES: Measles was virtually eliminated in South Africa following control activities in 1996/7. However, from July 2003 to November 2005, 1 676 laboratory-confirmed measles cases were reported in South Africa. We investigated the outbreak's cause and the role of HIV.
DESIGN: We traced laboratory-confirmed case-patients residing in the Johannesburg metropolitan (JBM) and O R Tambo districts. We interviewed laboratory- or epidemiologically confirmed case-patients or their caregivers to determine vaccination status and, in JBM, HIV status. We calculated vaccine effectiveness using the screening method.
SETTING: Household survey in JBM and O R Tambo districts.
OUTCOME MEASURES: Vaccine effectiveness, case-fatality rate, and hospitalisations.
RESULTS: In JBM, 109 case-patients were investigated. Of the 57 case-patients eligible for immunisation, 27 (47.4%) were vaccinated. Fourteen (12.8%) case-patients were HIV infected, 46 (42.2%) were HIV uninfected, and 49 (45.0%) had unknown HIV status. Among children aged 12 - 59 months, vaccine effectiveness was 85% (95% confidence interval (CI): 63, 94) for all children, 63% for HIV infected, 75% for HIV uninfected, and 96% for children with unknown HIV status. (Confidence intervals were not calculated for sub-groups owing to small sample size.) In O R Tambo district, 157 case-patients were investigated. Among the 138 case-patients eligible for immunisation, 41 (29.7%) were vaccinated. Vaccine effectiveness was 89% (95% CI 77, 95).
CONCLUSIONS: The outbreak's primary cause was failure to vaccinate enough of the population to prevent endemic measles transmission. Although vaccine effectiveness might have been lower in HIV-infected than in uninfected children, population vaccine effectiveness remained high.
Measles has recently been the focus of accelerated disease control activities in sub-Saharan Africa, resulting in a precipitous decline in reported cases and associated deaths.1 These activities were begun in the mid-1990s by seven African nations, including South Africa, and resulted in the virtual elimination of measles in southern Africa.2
South Africa has provided routine measles vaccination at 9 months of age since 1975. In 1995, the South African Department of Health (DoH) recommended that a second dose of measles vaccine be given at 18 months of age via routine health services, and set the goal of eliminating measles by 2002. To achieve this, South Africa used the strategy of the Pan American Health Organization.3 The first step was a nationwide measles vaccination campaign conducted in 1996 and 1997, targeting all children aged 9 months to 14 years, which achieved 85% administrative coverage. (Administrative coverage is the number of doses delivered divided by the target population. The target population is determined from census projections.) Subsequently, case-based surveillance with laboratory confirmation of suspected cases was established in South Africa. In 2000, a nationwide campaign targeting all children aged 9 months to 4 years reported 92% administrative coverage. From 1996 to 2004, routine measles first-dose coverage remained static at 76 - 83% while routine second-dose coverage was 63 - 78% from 2000 to 2004. Reported measles incidence reflected the reported success of the 1996 and 2000 campaigns; enhanced surveillance during 1999 - 2002 reported <60 measles cases annually and no associated deaths.1,2
However, measles virus was introduced in July 2003 to Mpumalanga and Gauteng provinces from Mozambique, which led to an epidemic lasting more than 2 years, with 1 676 reported laboratory-confirmed cases; 773 (46.1%) were from Gauteng and 506 (30.2%) from Eastern Cape; 27 measles-associated deaths were reported nationally. In Gauteng, 11 measles-associated deaths occurred in institutions for children; 8 were deaths of HIV-infected children.
In outbreak-affected provinces, teams investigated suspected measles cases and conducted response vaccination that targeted exposed children in preschools, schools and hospitals. In April 2004, Gauteng Province's DoH issued a directive to provide a supplemental dose of measles vaccine to all hospitalised and institutionalised children aged 6 months to 14 years. In addition, a national measles vaccination campaign targeting all children aged 9 months to 4 years was conducted in July 2004; reported administrative coverage was 102% in Gauteng but only 90% in Eastern Cape. Following this national campaign, measles virus transmission in Gauteng decreased, with the final case reported in May 2005. In contrast, measles transmission had not started in Eastern Cape until November 2004, months after the national campaign. To stop the spread of disease, a second province-wide vaccination campaign was conducted in Eastern Cape in May 2005, targeting children aged 6 months to 14 years and achieving 86% administrative coverage; nevertheless, measles virus transmission continued in the province until November 2005. This was the first large laboratory-confirmed measles outbreak following near-elimination in a setting with high HIV prevalence; in 2004, HIV seroprevalence in South Africa was estimated at 29.5% among antenatal clinic attendees.4 (HIV-infected children have a higher rate of primary measles vaccine failure than HIV-uninfected children.5) Questions have been raised as to whether the decreased efficacy of measles vaccine reported among HIV-infected children could affect the ability of high HIV-prevalence countries to achieve and sustain interruption of endemic measles virus transmission.5,6 We report on field investigations in two provinces to determine the cause of South Africa's measles outbreak and the role that HIV infection might have played in measles virus transmission.
Methods
We reviewed vaccination coverage data from 2000 to 2004 and case-based surveillance data for cases with date of rash onset falling between July 2003 and June 2005. Serum samples were collected for all suspected measles cases and sent to the National Institute for Communicable Diseases for analysis of measles IgM antibody using Enzygnost (Dade-Behring, Marburg, Germany) diagnostic kits.
Definitions
For surveillance purposes, the South African definition of a laboratory-confirmed measles case-patient (LCMCP) is a person with fever, rash, and measles IgM-positive serum. In addition, we defined an epidemiologically confirmed measles case-patient (ECMCP) as a person with fever, rash, and at least 1 of 3 symptoms (cough, coryza or conjunctivitis), who had had contact with a LCMCP. A measles-associated death was defined as death of a LCMCP or an ECMCP within 30 days of rash onset; this death must not have obviously resulted from another cause, e.g. trauma.7 A case-patient was considered vaccinated if the respondent provided documentation or history of receipt of one or more doses of measles-containing vaccine at least 30 days prior to rash onset. HIV status of a case-patient or case-patient's mother was determined by respondent-provided history or documentation of HIV test results. In South Africa, HIV PCR is available for children <15 months of age, and HIV ELISA is used to test older children and adults. Treatment with vitamin A was assessed by showing a vitamin A capsule to the respondent and asking how many capsules the case-patient had received during the illness.
Location
We selected two districts in which to conduct field investigations: the Johannesburg metropolitan district (JBM) in Gauteng province (the source of the largest absolute number of measles cases reported during the outbreak) and O R Tambo district in Eastern Cape province, which was the source of over half of all LCMCPs reported nationwide in 2005.
Gauteng province. From 25 May to 3 June 2005, we conducted an investigation of LCMCPs with rash onset between 1 July 2004 and 30 May 2005, residing in JBM and traced through available address information. We also investigated any household or school contacts meeting the ECMCP case definition identified during our investigation. We queried case-patients or their caregivers regarding the HIV status of the case-patient and the case-patient's mother, and obtained informed consent from all respondents.
Eastern Cape province. From 17 to 30 May 2005, a field investigation was conducted in O R Tambo district in all communities that reported LCMCPs with rash onset between 1 April and 30 May 2005. LCMCPs were interviewed and a house-to-house search was conducted in each case-patient's community to find ECMCPs with rash onset between 1 January and 30 May 2005.
Vaccine effectiveness
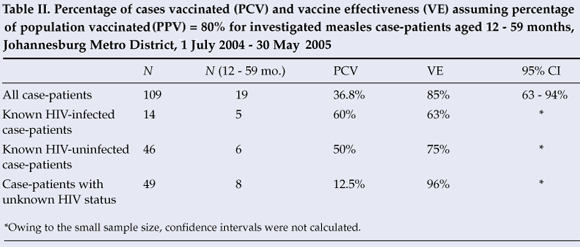
Vaccine effectiveness (VE) was calculated using the screening method with the formula VE=1-[PCV/(1-PCV)x(1-PPV)/PPV], where PCV refers to the percentage of cases vaccinated and PPV refers to the percentage of the population vaccinated.8 We confined our analysis to cases aged 12 - 59 months at the time of rash onset and our comparison population to this same age group, i.e. birth cohorts vaccinated in 2000 - 2004. Based on provincial routine measles first-dose vaccination coverage rates from 2000 to 2004, the PPV was estimated to be 80% in both Gauteng and Eastern Cape. Because some case-patients reported receiving vaccinations during campaigns only, we performed sensitivity analyses using an estimate of 90% as the PPV to account for the potential increase in the PPV following campaigns. We also calculated vaccine effectiveness among HIV-infected children, HIV-uninfected children, and children of unknown HIV status aged 12 - 59 months, assuming that there was no difference in age at vaccination or vaccination coverage between these groups.
Data analysis
Data were entered and analysed using EpiInfo v3.3.2 software (CDC, Atlanta, GA, USA). Calculation of vaccine effectiveness and confidence intervals was conducted using SAS 9.1 (SAS Institute, Cary, NC, USA). Confidence intervals were not calculated for sub-groups because of the small sample sizes.
Results
Gauteng province
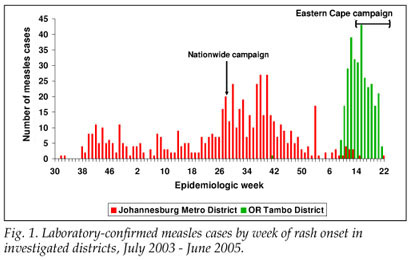
From 1 July 2004 to 30 May 2005, 454 LCMCPs were reported to the surveillance system from Gauteng province. Of these 454, 349 (76.9%) were reported from JBM (Fig. 1). Age distribution of the 349 LCMCPs from JBM is shown in Table I.
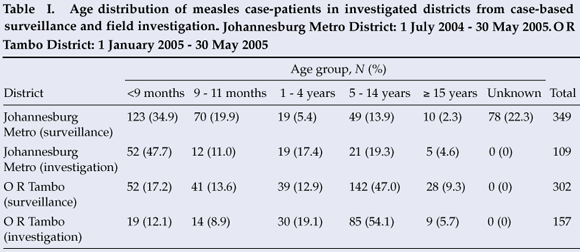
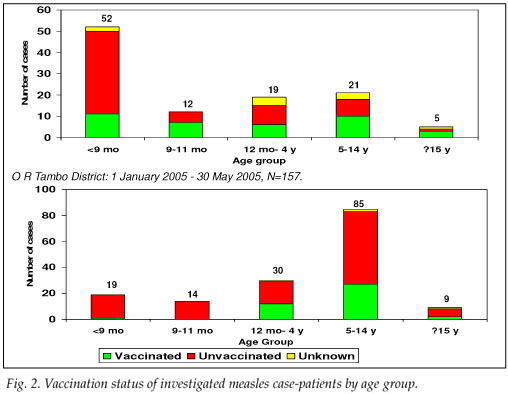
Address information to permit follow-up was available for 203 of the 349 LCMCPs. Among these 203, 2 refused consent, 107 were not found at the address given, and 94 were located and agreed to complete our questionnaire. While investigating the latter 94 cases, we identified an additional 15 ECMCPs for a total of 109 measles case-patients investigated (Table I). Fifty-seven (52.3%) case-patients reported having been hospitalised for measles, and 7 (6.4%) measles-associated deaths were reported to have occurred among the investigated case-patients. Among the 57 case-patients >9 months who were eligible for measles vaccination, 27 (47.4%) provided either documentation or a history of measles vaccination – leaving 52.6% of vaccine-eligible case-patients unvaccinated (Fig. 2).
Of the 109 investigated case-patients, 57 (52.3%) were hospitalised with measles and 44 (77.2%) reported having been isolated upon hospital admission. All 109 investigated case-patients visited health care facilities, and 48 (44%) reported having received >1 dose of vitamin A during their measles illness.
Among the 109 case-patients investigated, caregivers reported that 14 (12.8%) were HIV infected, 46 (42.2%) were HIV uninfected, and 49 (45.0%) did not know their HIV status. HIV-infected case-patients ranged in age from 3 months to 32 years. Twenty-nine (26.6%) case-patients' mothers were reported to be HIV infected, 47 (43.1%) were reported to be HIV uninfected, and 33 (30.3%) respondents did not know the HIV status of the case-patient's mother. Among the 83 case-patients aged <59 months at rash onset and therefore probably exposed to HIV only via maternal infection, caregivers reported that 9 (10.8%) were HIV infected, 40 (48.2%) were HIV uninfected, and 34 (41%) were of unknown HIV status. All 14 (100%) HIV-infected case-patients were hospitalised with measles, and 2 (14.3%) died. Twenty-eight (60.9%) of the 46 known HIV-uninfected case-patients were hospitalised, and 2 (4.3%) died. HIV-infected case-patients were 1.6 times more likely to be hospitalised than known HIV-uninfected case-patients (p=0.003). The difference in risk of death between HIV-infected case-patients and HIV-uninfected case-patients was not statistically significant (risk ratio (RR)=3.3, 95% CI 0.5, 21.2).
Among the 19 investigated case-patients aged 12 - 59 months, 7 (36.8%) were vaccinated. Vaccine effectiveness among all children aged 12 - 59 months was estimated to be 85% (95% CI: 63, 94) assuming the PPV to be 80% (Table II). Among the 5 HIV-infected case-patients in this age group, 3 (60%) were vaccinated, and vaccine effectiveness was estimated to be 63%. Three (50%) of the 6 HIV-uninfected case-patients were vaccinated and vaccine effectiveness was estimated to be 75%. One (12.5%) of 8 case-patients of unknown HIV status was vaccinated, and vaccine effectiveness was estimated to be 96%. If we assume that the campaign increased the PPV to 90%, vaccine effectiveness increased to 94% (95% CI 84, 97) for all children aged 12 - 59 months, 83% for HIV-infected children, 90% for HIV-uninfected children, and 98% for children of unknown HIV status.
Eastern Cape province
From 1 January to 30 May 2005, 417 LCMCPs were reported to the surveillance system from Eastern Cape province, of whom 302 (72.4%) were reported in O R Tambo district (Fig. 1). Information on age was available for all case-patients (Table I).
Seventy LCMCPs with rash onset between 1 April and 30 May 2005 and 87 ECMCPs identified during the field investigation were interviewed. Seventy-nine (50.3%) of these patients were hospitalised for measles, and 8 (5.1%) measles-associated deaths were reported among the investigated case-patients. Of the 138 case-patients >9 months at the time of rash onset and eligible for routine measles vaccination, 41 (29.7%) provided either documentation or a history of measles vaccination, leaving 70.3% of vaccine-eligible case-patients unvaccinated (Fig. 2). Among the 30 case-patients aged 12 - 59 months, only 9 (30.0%) provided documentation or a history of measles vaccination. Vaccine effectiveness in children aged 12 - 59 months was estimated to be 89% (95% CI 77, 95) in O R Tambo district, assuming the PPV to be 80%, and 95% (95% CI 90, 98) assuming the PPV to be 90%.
Among the 131 investigated case-patients who visited health care facilities, 60 (45.8%) received >1 dose of vitamin A during their measles illness. Among the 79 case-patients who were hospitalised, 46 (58.2%) reported having been placed in isolation.
Discussion
The primary cause of the measles outbreak in South Africa was the accumulation of susceptible children due to low routine immunisation coverage: 65% of vaccine-eligible case-patients interviewed in two districts born since the initial 1996 national campaign reported no measles vaccination in routine services or campaigns. This finding is consistent with reports of routine immunisation coverage of <83% since 2000. Although follow-up campaigns in 2000 and 2004 reported 92% administrative coverage nationally, these figures might have overestimated true coverage owing to inaccurate census estimates of the target populations. This tendency mirrors experience from the Americas, where relatively low routine and campaign coverage following years without measles transmission led to large outbreaks once the virus was reintroduced,9 which emphasises the importance of maintaining a very high population immunity even in the absence of a circulating virus.10
Our investigations supported national surveillance data in finding a substantial difference in the age distribution of measles case-patients between the two districts (Table I). The JBM outbreak primarily affected children <1 year old, while the O R Tambo outbreak affected children aged 5 - 14 years. Factors that could have lowered the age of measles acquisition in JBM district include high population density, poor maternal antibody transfer in HIV-exposed infants, and outbreaks in children's institutions.11 In O R Tambo district, historically low routine measles vaccination coverage and poor campaign performance might have left more school-age children susceptible to measles virus.
The World Health Organization (WHO) and United Nations Children's Fund (UNICEF) Integrated Management of Childhood Illness guidelines recommend 2 doses of vitamin A given 24 hours apart to reduce measles mortality.12 However, in this investigation, less than half of the children presenting to health facilities reported receiving any vitamin A. Isolation of suspected measles cases limits nosocomial measles transmission.13 Measles transmission probably occurred in institutions for children and health facilities in Gauteng province, as 58 measles cases were reported from these institutions, and isolation measures in the health facilities visited were inadequate. As with previous measles outbreaks, there is considerable need for more extensive therapeutic use of vitamin A and greater use of respiratory isolation.13,14
Maternal HIV infection might have contributed to measles virus transmission in JBM via reduced placental measles IgG transfer.11 The maximum expected HIV prevalence for the population of children aged <5 years in Gauteng province is 10%, given the 2004 antenatal clinic prevalence estimates of 33.1% in Gauteng (case-patient), and assuming 30% mother-to-child transmission.15 Prevention-of-mother-to-child-transmission (PMTCT) programmes might have reduced mother-to-child HIV transmission further in Gauteng,16 and higher mortality among HIV-infected children might also have reduced the proportion of HIV-infected children in the province. However, because we used facility-based surveillance to identify case-patients, HIV-infected measles case-patients might have been over-represented. Therefore, the rate of reported HIV infection of 10.8% among measles case-patients from JBM may reflect increased measles prevalence or morbidity in children with HIV, a bias in case finding, or a mix of these and other factors.
HIV infection among case-patients might have contributed to the morbidity and mortality associated with the Gauteng outbreak; 100% of the patients we investigated who were known to be HIV infected were hospitalised, compared with only 60.9% of known HIV-uninfected patients. This was the only statistically significant finding suggestive of an increase in measles morbidity attributable to HIV infection. Our higher case fatality rate (CFR) among HIV-infected patients was not statistically significant but is consistent with other findings of increased measles CFR in HIV-infected children.17 The decreased point estimate of vaccine effectiveness among HIV-infected children of 63% is consistent with reported estimates of lower measles vaccine effectiveness among HIV-infected children. Despite the possible decrease in vaccine effectiveness among HIV-infected children, overall vaccine effectiveness in Gauteng province remained relatively high at 85%, which suggests that decreased vaccine effectiveness in HIV-infected children should not pose a threat to regional or global measles mortality reduction goals and is consistent with modelling studies.18
For VE estimates, we chose to use a PPV of 80%. This PPV, based upon reported one-dose coverage, probably underestimates the true proportion of the population vaccinated. The primary purpose of calculating VE in our investigation was to ascertain the minimum VE. As demonstrated by our sensitivity analysis, a higher estimate of PPV would result in higher VE, therefore supporting our conclusion that vaccine failure did not play a substantial role in the outbreak.
Despite the activities of outbreak response teams, measles virus transmission continued until province-wide measles campaigns had been conducted. Vaccination of contacts alone has been shown to be ineffective in stopping transmission of measles in resource-limited settings.19 Prompt initiation of a wide age-range campaign in affected districts or provinces may be a more effective use of resources in halting measles transmission.20 Factors that might have resulted in shorter duration of the outbreak in Eastern Cape than in Gauteng include lower population density and earlier intervention with a wide age-range campaign.
There were several limitations to these field investigations. Firstly, small sample sizes made it difficult to detect differences among sub-groups and might have increased the effect of misclassification on VE estimates. Secondly, surveillance data from health facilities formed the basis of our investigations; but cases presenting to health facilities might not have been representative of all measles cases, particularly regarding age and HIV status. Thirdly, we were unable to locate more than half of the LCMCPs in Gauteng. Lastly, HIV status and vaccination status were determined by respondent report, and responses might have been affected by a social desirability bias. Additionally, unknown HIV status might have been a better marker of lack of access to care than HIV status, as only 1 child in this subgroup had been vaccinated prior to the outbreak. Mothers of infants of unknown HIV status were also more likely to be of unknown HIV status. Consequently, the increased VE among children with unknown HIV status is probably a mathematical artefact of the screening method, driven by a low PCV of 12.5%.
Conclusions
Our investigations showed the importance of maintaining high population immunity by means of routine immunisation services to prevent transmission following importation of the measles virus. Although HIV infection in measles case-patients might have contributed to outbreak-associated morbidity and mortality, high population vaccine effectiveness was maintained. Our investigation also revealed that vitamin A and respiratory isolation continue to be underutilised in measles outbreaks, and that case investigation with contact vaccination is not effective in stopping measles virus transmission.
We gratefully acknowledge the contributions of the Department of Health of the Republic of South Africa, the World Health Organization office in South Africa, the World Health Organization Regional Office for Africa Southern Bloc ICP, the National Institute for Communicable Diseases (NICD) and the Children's Homes Outreach Medical Program (CHOMP). We thank the following individuals: Mrs Estelle de Klerk, Dr Nthombenhle Ngcobo, Mrs Lindsay Botham, Ms Nthabeleng Motsomi, Dr Dan Kibuuka, Ms Thandi Chaane, Mrs Joy Mnyaluza, Mrs Antonia Barnard, Mr Alan Wild, Mrs Mvulakazi Thipanyane, Dr W Shasha, Mr Akpan Etukudo, Mr Eric Wiesen, Professor Barry Schoub, Mrs Jo McAnerney and Dr Michelle Meiring. We also thank Mr Thomas H Taylor Jr, in the Office of the Chief Science Officer at the US Centers for Disease Control and Prevention, for his assistance with statistical analysis.
References
1. Otten M, Kezaala R, Masresha B, et al. Public-health impact of accelerated measles control in the WHO African Region 2000-2003. Lancet 2005; 366: 832-839. [ Links ]
2. Biellik R, Madema S, Taole A, et al. First 5 years of measles elimination in southern Africa: 1996-2000. Lancet 2002; 359: 1564-1568. [ Links ]
3. de Quadros CA, Olive JM, Hersh BS, et al. Measles elimination in the Americas – evolving strategies. JAMA 1996; 275: 224-229. [ Links ]
4. Department of Health. National HIV and syphilis antenatal sero-prevalence survey in South Africa 2004. Pretoria: Department of Health, 2005. http://www.doh.gov.za/docs/reports/2004/hiv-syphilis.pdf (accessed 14 April 2006). [ Links ]
5. Palumbo P, Hoyt L, Demasio K, Oleske J, Connor E. Population-based study of measles and measles immunization in human immunodeficiency virus-infected children. Pediatr Infect Dis J 1992; 11: 1008-1014. [ Links ]
6. Moss WJ, Clements CJ, Halsey NA. Immunization of children at risk of infection with human immunodeficiency virus. Bull World Health Organ 2003; 81: 61-70. http://www.who.int/bulletin/Moss0103.pdf (accessed 14 April 2006). [ Links ]
7. World Health Organization. Generic protocol for determining measles case fatality rates in a community, either during an epidemic or in a highly endemic area. WHO document WHO/EPI/GEN/93.3. Geneva: WHO, 1993. [ Links ]
8. Farrington CP. Estimation of vaccine effectiveness using the screening method. Int J Epidemiol 1993; 22: 742-746. [ Links ]
9. Centers for Disease Control and Prevention. Outbreak of Measles – Venezuela and Colombia, 2001-2002. MMWR Morb Mortal Wkly Report 2002; 51: 757-760 http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5134a1.htm (accessed 14 April 2006). [ Links ]
10. Orenstein WA, Hinman AR, PJ Strebel. Measles: the need for 2 opportunities for prevention [commentary]. Clin Infect Dis 2006; 42: 320-321. [ Links ]
11. Scott S, Cumberland P, Schulman CE, et al. Neonatal measles in rural Kenya: The influence of HIV and placental malaria infections on placental transfer of antibodies and levels of antibody in maternal and cord serum samples. J Inf Dis 2005; 191: 1854-1860. [ Links ]
12. D'Souza RM, D'Souza RD. Vitamin A for the treatment of children with measles – a systematic review. J Trop Pediatr 2002; 48: 323-327. [ Links ]
13. Biellik RJ, CJ Clements. Strategies for minimizing nosocomial measles transmission. Bull World Health Organ 1997; 75: 367-375. http://whqlibdoc.who.int/bulletin/1997/Vol75-No4/bulletin_1997_75(4)_367-375.pdf (accessed 14 April 2006). [ Links ]
14. Nandy R, Handzel T, Zaneidou M, et al. Case-fatality rate during a measles outbreak in eastern Niger in 2003. Clin Infect Dis 2006; 42: 322-328. [ Links ]
15. Nolan ML, Greenberg AD, Fowler MG. A review of clinical trials to prevent mother-to-child HIV-1 transmission in Africa and inform rational intervention strategies. AIDS 2002; 16: 1991-1999. [ Links ]
16. Coetzee D, Hilderbrand K, Boulle A, Draper B, Abdullah F, Goemaere E. Effectiveness of the first district-wide programme for the prevention of mother-to-child transmission of HIV in South Africa. Bull World Health Organ 2005; 83: 489-494. http://www.who.int/bulletin/volumes/83/7/489.pdf (accessed 14 April 2006). [ Links ]
17. Oshitani H, Suzuki H, Mpabalwani ME, Mitzuta K, Numazaki Y. Measles case fatality by sex, vaccination status and HIV-1 antibody in Zambian children. Lancet 1996; 348: 415. [ Links ]
18. Helfand RF, Moss WJ, Harpaz R, Scott S, Cutts F. Evaluating the impact of the HIV pandemic on measles control and elimination. Bull World Health Organ 2005; 83: 329-337. http://www.who.int/bulletin/volumes/83/5/329.pdf (accessed 14 April 2006). [ Links ]
19. Quiroga R, Barrezueta O, Venczel L, et al. Interruption of indigenous measles transmission in Bolivia since October 2000. J Infect Dis 2003; 187 (suppl 1): S121-126. [ Links ]
20. Pan American Health Organization. Update: Sao Paulo Measles Outbreak. PAHO EPI Newsletter 1998; 20: 5-6. http://www.paho.org/english/ad/fch/im/sne2001.pdf (accessed 14 April 2006). [ Links ]
Accepted 3 November 2008.
* Corresponding author: M McMorrow (MMcmorrow@cdc.gov)














