Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.99 no.4 Pretoria Abr. 2009
ORIGINAL ARTICLES
Guideline for the diagnosis, prevention and treatment of paediatric ventilator-associated pneumonia
B M MorrowI; A C ArgentI,II; P M JeenaIII; R J GreenIV
IPhD. Division of Paediatric Critical Care and Children's Heart Disease, School of Child and Adolescent Health, University of Cape Town and Red Cross War Memorial Children's Hospital, Rondebosch
IIFCPaed (SA). Paediatric Intensive Care Unit, Red Cross War Memorial Children's Hospital, Rondebosch
IIIFCPaed (SA), Cert Pulm (Paed). Division of Paediatric Pulmonology and Intensive Care, Department of Paediatrics and Child Health, University of KwaZulu-Natal and Inkosi Albert Luthuli Hospital, Durban
IVFCPaed (SA), PhD. Division of Paediatric Pulmonology and Intensive Care, Department of Paediatrics and Child Health, University of Pretoria and Steve Biko Academic Hospital, Pretoria
ABSTRACT
OBJECTIVE: Ventilator-associated pneumonia (VAP) has been poorly studied in South Africa, but is likely to be a significant problem, with resulting increased morbidity and mortality in the paediatric intensive care unit population. This guideline is intended to review the evidence and recommendations for prevention and management of VAP in children and to provide, where possible, clear advice to aid the care of these children, to limit costly and unnecessary therapies and - importantly - limit inappropriate use of antimicrobial agents.
EVIDENCE: The Working Group was constituted. Literature on the aetiology, prevention and management of paediatric VAP is reviewed.
RECOMMENDATIONS: Evidence-based clinical practice guidelines are provided for VAP diagnosis and prevention in South Africa. In addition, the current status of antimicrobial use has been reviewed and clear recommendations are set out.
1. Background
Ventilator-associated pneumonia (VAP) has been defined as a nosocomial lower respiratory tract infection occurring in mechanically ventilated patients 48 hours or more after initiation of ventilatory support.1 However, the precise definition according to clinical, pathological and/or microbacterial criteria is unclear, with little validation in the paediatric age group and no consistency of application between centres.
The gold standard for the diagnosis of pneumonia in adults is histological examination of lung tissue. Until recently, however, the recognition of histological pneumonia varied between pathologists2 and there was a discrepancy between the bacterial density of cultures taken via the airway and histological features of pneumonia.3 This could be expected, as the histological changes would depend on the infection and also the response mounted by the patient. Although it is unlikely that a universally applicable and acceptable definition of VAP in children will be established in the near future, there would be substantial advantages if centres in South Africa could collect data related to an agreed definition. Primarily, this would establish a database that could allow assessment of the extent of the problem and response to chosen intervention programmes.
VAP has been associated with increased length of ventilator dependence; increased paediatric intensive care unit (PICU) and hospital stay; increased mortality; and increased hospital costs.4 At the Red Cross War Memorial Children's Hospital (RCH) in Cape Town, it was found that, despite a similar initial prognosis and illness severity, patients who developed VAP had a significantly higher overall mortality rate; a trend towards increased PICU mortality; a 56% and 43% increase in the length of PICU and hospital stay respectively; and almost double the duration of ventilatory support.5
VAP has been little studied in South Africa, with only 2 papers published in the last 10 years relating to nosocomial infection in PICUs.5,6 Yet reduction of VAP would improve patient outcome, reduce costs, and increase the number of PICU beds available to new patients.5 These effects could be extremely significant in an environment of limited resources, relatively high mortality and severe shortage of PICU beds.
Patients admitted to PICUs in South Africa differ substantially from those in developed countries. The average age of children requiring mechanical ventilation at RCH is <6 months,5 whereas PICU patients in developed countries are generally >2 years of age.7,8 In South Africa, patients are most commonly admitted to PICU for the management of infection (mainly pneumonia and gastro-enteritis)5 whereas in developed countries the major reason is care after surgical procedures.7,8 In developed countries, immunodeficiency is uncommon7 whereas South Africa has a very high HIV prevalence. In addition, poor socio-economic circumstances and poor access to health care9 may delay presentation to tertiary institutions, so that patients are more severely ill on admission. In developed countries, the main reasons for childhood mortality are related to prematurity, congenital heart disease, malignancy and trauma10 whereas, in South Africa, children die predominantly as a result of HIV/AIDS, diarrhoeal disease and lower respiratory tract infections.11 A systematic review of VAP in adults in developing countries concluded that the incidence of VAP is higher than benchmarks from the USA, with a significant impact on patient outcome.12
For these reasons, it is not appropriate to apply studies and clinical guidelines from developed countries to clinical practice in South Africa. However, it is important to develop appropriate guidelines for use in our resource-constrained environment to ensure optimal utilisation of scarce resources, including the small number of PICU beds servicing a large paediatric population.
This paper provides clinical practice guidelines, graded according to the strength of the evidence levels (Table I).
2. Definitions and diagnosis
Consistent definitions should be used countrywide (ideally, worldwide) to provide a common baseline for clinical practice and research. The most commonly used definitions of VAP are those published by the Centers for Disease Control,13 but these are complex and not easily applied in South Africa.
2.1 Clinical pulmonary infection score
The clinical pulmonary infection score (CPIS) (Table II) rates various clinical and radiographic signs from 0 to 2, with a total score of >6 indicating a high probability of VAP.14 Compared with quantitative bronchoalveolar lavage (BAL) cultures, the CPIS has low sensitivity and specificity for diagnosing VAP in adults, and poor inter-observer reliability.15 The CPIS has not been validated in the paediatric age group.
Recommendation: A quantitative scale is useful for a clinical audit in ensuring a standardised diagnostic tool, but the CPIS first requires validation and adaptation for the paediatric age group (level of evidence: D (no paediatric data)).
Clinical criteria for paediatric VAP have been suggested and are summarised in the boxed inserts below.16 Positive BAL culture may be substituted for one clinical criterion.1
The value of each of these individual clinical criteria is questionable, as will be discussed.
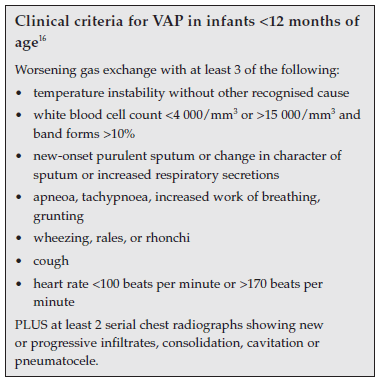
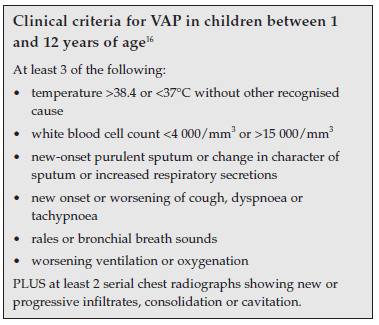
2.2 Sequential abnormal chest radiographs
Using this criterion necessitates routine daily chest radiographs, which are expensive, have no clear benefit to the patient17 and are potentially harmful owing to unnecessary radiation exposure.18 In addition, the analysis of chest radiographs may not be accurate because of:
• interpretation; for example, differentiating atelectasis from consolidation can be difficult,16 and only the presence of consolidation is highly correlated with the specific diagnosis of airspace disease19
• different radiograph exposures, which may lead to inaccurate assessments of change
• lack of significant radiograph findings even with severe infection in immunocompromised patients - a particular concern in our highly HIV-prevalent environment16
• high positive end-expiratory pressure (PEEP) levels in ventilated patients, which may give the impression of resolution of infiltrates because of an increase in radiographic translucency20
• presence of cardiac failure, and extensive fluid retention which may occur in patients with severe sepsis and/or renal failure.
Based on a systematic review of adult and paediatric data, there is insufficient evidence to support the practice of either routine or restrictive radiography.21
Recommendation: Chest radiographs should be taken on clinical suspicion (restrictive practice) in order to reduce costs, prevent unnecessary treatment based on spurious signs, and minimise radiation exposure to the patient (level of evidence: B (non-randomised study of >3 500 PICU patients)).
2.3 Fever
Fever >38.4ºC15,16 may be a rare event in a PICU environment because any elevation in temperature is usually actively treated. The site of measurement influences the accuracy and interpretation of the temperature measurement;22 therefore, this should be recorded in studies of paediatric VAP and preferably only core temperature be used.23 Temperature instability or fever can only be a valid clinical criterion if measures are not taken to actively prevent or manage fever. Therefore, if this is a requirement, studies should report that no servo controllers were used, no paracetamol was given and no tepid sponging was done by nursing staff.
Recommendation: The site and method of temperature measurement must be recorded on all VAP audits/studies. Active management of fever precludes this clinical criterion (level of evidence: D).
2.4 Infectious markers
Severe infections of infants and children must be identified and treated promptly to optimise patient outcome.24 Non-bacterial infections must be recognised early to avoid unnecessary antibiotic use. Bacterial pneumonia cannot be differentiated from viral pneumonia on the basis of clinical characteristics, routine laboratory tests, or chest radiographs, and it is unclear whether white blood cell concentration or serum C-reactive protein (CRP) concentration consistently differentiates between them.24 Leucopenia or leucocytosis have been suggested as clinical criteria for the diagnosis of VAP. The CPIS (Table I) includes the presence of neutrophil band forms in its criteria. The addition of an abnormal procalcitonin (PCT) may be useful as an accurate and early marker of severe bacterial infection in children.24
Recommendation: Leucopenia/leucocytosis, neutrophil band forms and PCT should be included in the diagnosis of VAP. It may also be helpful to indicate whether septic markers are increasing or decreasing, considering the high infectious load of South African PICU patients (level of evidence: D).
2.5 Sputum production, work of breathing and auscultation
Sputum quantity and quality are poor outcome measures, even in controlled study environments.25 Secretions are influenced by the time of day,25 presence and degree of humidification of ventilator gases, frequency of suctioning, and saline instillation.
Increased work of breathing, tachypnoea and apnoea may not be apparent in a ventilator-dependent child, especially if heavily sedated. The interpretation of pulmonary sounds on auscultation is subjective and unreliable in infants26 and is highly dependent on experience and hearing ability.27
Recommendation: Sputum quality and quantity, work of breathing, and auscultation findings are subjective outcome measures. Together they may indicate a change in clinical status, but there is no evidence to support this (level of evidence: D).
2.6 Clinical v. microbiological diagnosis of VAP
Research is required to develop and validate an appropriate clinical diagnostic score for paediatric VAP for use in South Africa. Until such studies are available, reports, studies and audits should state exactly which criteria were used, to improve internal and external validity; preferably, the same criteria should be used to allow comparison of data between centres (see recommendations below).
Clinical criteria are 100% sensitive but poorly specific (15%) for the diagnosis of VAP in adults28 and may therefore overestimate the incidence of VAP if used in isolation.14,29 Blind BAL has 73% sensitivity and 96% specificity for the diagnosis of VAP28 and, therefore, by combining sensitive (clinical) and specific (BAL) tests, good diagnostic validity should be achieved.28
Blood culture results in childhood pneumonia are frequently negative30 and therefore generally not helpful in the diagnosis of VAP, although a positive result will guide therapy. Over-treatment may occur on the basis of results of respiratory specimens with poor specificity, such as endotracheal aspirates.28 It is therefore recommended that, wherever possible, lower respiratory tract specimens (BAL) should be performed on admission and thereafter if there is a clinical indication to change therapy. This strategy could prevent the indiscriminate use of antibiotic coverage in all patients who develop signs and symptoms suggestive of pneumonia, thereby minimising the emergence of resistant organisms.29 Although non-bronchoscopic BAL has complications in the South African paediatric population,31 its risks must be weighed against the benefits for the individual and the community of identifying true pathogenic organisms. Risks of BAL can be significantly reduced by using a simple adaptation of the endotracheal tube.32
Recommendation: Blood culture results may be included in the diagnostic workup for VAP, but a negative result does not exclude VAP. Clinical signs, which have good sensitivity but poor specificity, should be used in conjunction with a highly specific test such as BAL. Where possible, invasive respiratory specimens should be taken rather than wasting precious resources to obtain frequent, poor-quality specimens from the upper respiratory tract (level of evidence: B). To ensure standardisation, it is suggested that a modified CPIS be used for the diagnosis of VAP, replacing quantitative tracheal aspirate culture with positive BAL culture,5 as none of the paediatric criteria has been validated, and using the CPIS will include the majority of appropriate signs in the clinical criteria suggested by Wright and Romano.16
3. Aetiology
Aspiration may be an important cause of VAP in children,7,8,33 and prolonged mechanical ventilation,4 genetic syndromes, transport into and out of the PICU, re-intubation,8 prior antibiotic use, continuous enteral feeding, bronchoscopy8 and immunodeficiency33 have all been identified as independent predictors of VAP. Prior use of carbapenems and third-generation cephalosporins are independent risk factors for acquisition of multidrug-resistant (MDR) Acinetobacter baumannii.34
A. baumannii was the most common organism isolated from bronchoalveolar lavage specimens in VAP-defined patients in a South African PICU,5 followed by Klebsiella pneumoniae, Staphylococcus aureus and Pseudomonas aeruginosa. Jeena et al.6 reported the emergence of MDR Acinetobacter spp. in South African neonatal and paediatric units, associated with >50% mortality and significant morbidity. These results are similar to a Brazilian study - a developing country with some challenges common to those of South Africa.35 In an Indian paediatric VAP study, Escherichia coli and K. pneumoniae were isolated most commonly, followed by P. aeruginosa, Proteus spp. and A. baumannii.36 It is difficult to distinguish between endotracheal tube (ETT) colonisation and true pathogenic organisms in both of these studies, as the first-mentioned did not clearly describe the method of obtaining respiratory specimens and the latter only cultured the ETT tip. Studies from developed countries have implicated P. aeruginosa, K. pneumoniae and S. aureus as the most common causative organisms in paediatric VAP.7 P. aeruginosa and S. aureus were the most common organisms cultured in a Saudi Arabian PICU,8 which is well-resourced relative to developing countries.
Non-bacterial organisms such as yeasts35,37 and viruses have also been implicated in paediatric VAP. Respiratory syncytial virus (RSV) has been identified as an important but under-recognised cause of nosocomial infection among children in South Africa38 and was responsible for almost 15% of VAP cases in a South African PICU.5 Outbreaks of adenovirus infections have also been reported from South Africa, with a high associated mortality in one study.39,40 In RCH PICU, one case of presumed nosocomial Pneumocystis jiroveci pneumonia (PJP) acquisition was reported in a child with non-HIV comorbidity.5 Nosocomial transmission of P. jiroveci has been reported in adults41,42 and may be a concern in PICUs with high HIV prevalence.
4. Prevention
4.1 Infection control
In the USA, about one-third of all nosocomial infections could be prevented by strict adherence to existing infection control policies.43 Hospital staff have been implicated as a transmission source of nosocomial infections, largely as a result of inadequate or poor hand-washing technique.44 High patient-to-staff ratios significantly influence the ability of staff to adhere to basic infection control procedures45,46 and are significantly associated with a high incidence of VAP.46-48 Therefore, South African practitioners should lobby for more and better-trained PICU nurses. Considering that A. baumannii, which is capable of surviving on surfaces for extended periods, has been implicated in South African paediatric VAP,5 it is essential to thoroughly and regularly decontaminate, in addition to standard infection control measures.44
Recommendation: Effective infection control practices, including hand and environmental decontamination, are the most important preventive strategy for nosocomial infection, including VAP (level of evidence: A).
4.2 The 'bundle' approach
The Prevention of VAP bundle of care, developed by the Institute for Healthcare Improvement (IHI), was developed for adult patients, but has been applied to paediatric practice. This bundle includes: (i) elevation of the bed to 30 - 45 degrees; (ii) daily sedation vacation and daily assessment of readiness to extubate; (iii) peptic ulcer prophylaxis; and (iv) deep-vein thrombosis (DVT) prophylaxis.49 Additional interventions such as oral hygiene, closed-system/inline suctioning and oro- rather than nasotracheal intubation have also been advocated.49,50
4.3 Head-of-bed elevation
A RCT of 86 adult ventilated patients showed that the incidence of VAP was significantly lower in those positioned in a semi-recumbent position compared with patients lying supine,51 probably because of decreased gastro-oesophageal reflux (GOR) and aspiration.52,53
There are no similar trials in the paediatric age group; however, it is probable that children and infants have the same, if not increased, risk as adults of GOR and aspiration.49 Head-of-bed elevation is a low-risk intervention which is likely to hold risks only for patients with specific cardiac disorders or severe sepsis, and is therefore recommended in PICU. However, it is not known what angle of inclination is optimal or achievable in this age group. In infants, a reverse Trendelenburg position may be used with bassinettes and open incubators.49 In addition to head-of-bed elevation, post-pyloric feeding is recommended for infants at high risk of GOR and aspiration,49,54 although there is no objective evidence to support this.
Recommendation: PICU patients should be nursed in the head-up or reverse-Trendelenburg position (level of evidence: B (extrapolated from adult RCT). Post-pyloric feeding should be used for infants at risk of GOR (level of evidence: D).
4.4 Daily sedation vacation and daily assessment of readiness to extubate
Prolonged mechanical ventilation is a risk factor for paediatric VAP,4,36 so all available measures to reduce the duration of ventilation should be taken.
RCTs in ventilated adult patients indicate that a 'wake up and breathe' protocol, which involves interrupting sedatives and allowing spontaneous breathing (ventilator weaning), results in reduced duration of mechanical ventilation, ICU stay and mortality. It has therefore been recommended as standard practice for adult ventilated patients.55,56 These 'sedation vacations' are not appropriate for children and infants as the PICU is a foreign and frightening environment for a non-sedated child, inadequate sedation is a risk factor for accidental extubation,57 and re-intubation increases the risk of VAP.7
Paediatric studies suggest that children are being over-sedated in PICUs,58-60 which may contribute to weaning failure.61 Therefore, appropriate levels of sedation should be maintained such that ideally the child is awake but comfortable and able to breathe spontaneously. Continuous heavy sedation should be avoided as this depresses the cough reflex and spontaneous ventilation and predisposes to aspiration of oropharyngeal secretions.49
Clinicians should routinely evaluate PICU patients' readiness to extubate rather than routinely weaning their patients off ventilator support, as routine weaning is likely to prolong the ventilation time of those ready for extubation.61
Recommendations: 'Sedation vacations' are not appropriate for paediatric practice, but sedation levels should be monitored and kept at minimal levels (level of evidence: D). Clinicians should routinely assess the paediatric patient's readiness for extubation instead of implementing weaning protocols (level of evidence: A (RCT of 182 infants and children61)).
4.5 Peptic ulcer prophylaxis
Acidification of gastric contents is thought to decrease colonisation with potentially pathogenic bacteria. Conversely, increasing gastric pH (as would occur when using histamine-2-receptor (H2)-antagonists and antacids as stress ulcer prophylaxis) may increase colonisation, thereby predisposing to VAP.62 Sucralfate is an alternative agent that does not change gastric pH, and it was therefore postulated that it would also decrease the incidence of VAP.
A retrospective study of 155 paediatric patients ventilated for >48 hours showed no significant differences in the incidence of VAP between patients treated with sucralfate or ranitidine.63 A prospective RCT of 160 PICU patients, which assigned them to treatment with ranitidine, omeprazole or sucralfate, or no treatment, found no difference in the incidence of VAP, macroscopic stress ulcer bleeding, or mortality between the arms of the study.64
These studies might have been underpowered to detect a true difference between patients treated with different agents, but stress ulcer prophylaxis may not be associated with VAP in the paediatric age group.4 Despite the assertion that peptic ulcer prophylaxis is considered to be a standard of PICU care,49 there are insufficient data to support its routine use in paediatric practice.
Recommendation: Stress ulcer prophylaxis should not be routinely used in the PICU (level of evidence: A (paediatric RCT).
4.6 DVT prophylaxis
There are no data on the impact of DVT prophylaxis on VAP in adults or children; nevertheless, DVT prophylaxis is included in the 'ventilator bundle' as 'excellent practice'.49 Limited data exist on the risks of DVT in children; it is therefore suggested that patients be individually assessed according to their likely risk of developing DVT.65
Recommendation: DVT prophylaxis should be administered based on individual risk assessments, but should not be part of a VAP preventive 'bundle' (level of evidence: D).
4.7 Other interventions
4.7.1 In-line suctioning
Some guidelines recommend using in-line or closed-system suctioning (CSS)49 instead of open endotracheal suctioning, based on the postulation that CSS would reduce the incidence of VAP by eliminating environmental contamination of the catheter before introduction into the endotracheal tube.66 However, CSS is associated with significant microbial colonisation of the respiratory tract, and bacterial growth on the catheter itself, particularly if the CSS catheter is not changed for extended periods.67,68 CSS has also been reported to be less effective at clearing secretions than open suctioning,69,70 and is costly. The cost of a single CSS system in South Africa is likely to be R170 (≈$17.90) per system v. R1 - R2 (≈$0.10 - $0.20) per catheter used in open suctioning. However, the human resource cost savings for CSS should be borne in mind.
Meta-analyses have found no significant differences between open suctioning and CSS on the incidence of VAP and mortality in adults.68,71,72 Paediatric data are limited, but a RCT of 175 low-birth-weight infants showed that CSS did not affect the rate of bacterial airway colonisation, frequency of endotracheal suctioning and re-intubation, duration of mechanical ventilation, length of hospitalisation, incidence of nosocomial pneumonia or neonatal mortality.73
Recommendation: Clinicians should continue to use the suction method with which they are proficient; there is no benefit from closed or open suctioning systems on the incidence of VAP (level of evidence: B (extrapolated from adult meta-analyses and neonatal RCT)).
4.7.2 Oral hygiene
In adults, dental plaque may become colonised with potentially pathogenic organisms,74 which may predispose to VAP. Meticulous oral hygiene reduces the incidence of VAP in adults,75 as does oral decontamination with chlorhexidine.76
The age-related pattern of bacterial colonisation is connected with the development of dentition,77 but no studies have related this to the development of VAP in children. There are also no paediatric data on the effects of oral hygiene on VAP, but it seems advisable to follow the recommendations of wiping gums with gauze in the absence of dentition,49 as some commensals are able to adhere to epithelial surfaces in edentulous infants.77 Bacterial colonisation increases and becomes established after the primary dentition emerges, from about 6 months of age, as the teeth provide attachment sites for oral bacteria.77 Therefore, where teeth are present, they should be brushed with toothpaste if it is possible to do so, and regular oropharyngeal cleaning should be performed with a mouthwash.49 Chlorhexidine has been recommended on the basis of adult data76 but, because of its unpleasant taste, a more palatable alternative should perhaps be considered for infants and children.
Recommendation: Regular oral hygiene should be implemented in children (level of evidence: B (extrapolated from adult RCT)).
4.7.3 Orotracheal v. nasotracheal intubation
The link between nosocomial sinusitis and VAP was suggested by a randomised study of 399 nasotracheally intubated adults in whom the incidence of VAP and mortality was significantly lower when sinusitis was actively sought and treated.78
It has been widely suggested that naso-endotracheal tubes should be avoided owing to the increased risk of nosocomial sinusitis;49,79 however, the literature is not clear on this topic. Bach et al.80 reported a significantly greater risk of nosocomial sinusitis with nasal rather than oral intubation in a RCT of 68 ICU patients. In contrast, Holzapfel et al.,81 in a RCT of 300 adult patients, showed no significant differences in time to occurrence of nosocomial sinusitis, pneumonia, septicaemia or overall survival rate between the two types of intubation. Because of slight differences between study designs, one cannot conclude for certain that nasotracheal intubation causes nosocomial sinusitis.82
There are many potential contributing factors other than nasal intubation to the development of nosocomial sinusitis while in the ICU. Tubes of smaller diameter (such as nasogastric feeding and suction tubes) can significantly obstruct the normal flow of sinus fluids, leading to an increased risk of bacterial colonisation and development of nosocomial sinusitis.83 Heavy sedation is another important risk factor,83 as normal clearance mechanisms such as coughing and sneezing are suppressed.84 The recumbent position may also increase nasal congestion and cause obstruction of the maxillary sinus ostia.84
The risk of nosocomial sinusitis in ventilated children and infants has not been assessed. There is a known risk of airway complications when using oral intubation,79 possibly more so in children than adults. Other potential consequences of oral intubation in infants and children include conditioned dysphagia, which may be caused by multiple medical procedures occurring around the face and mouth.85 There was a higher incidence of tracheal aspiration of pharyngeal contents (a risk factor for VAP) with oral endotracheal tubes, in a study from Brazil.86
There is insufficient evidence to support oral or nasal methods of intubation in paediatric practice. In PICUs with staff shortages, as in developing countries such as South Africa, consideration should be given to the increase in workload necessary to prevent accidental extubation as a result of potentially unstable oral endotracheal tubes in minimally sedated patients.
Recommendation: Clinicians can use either oral or nasal intubation, but must be aware of the potential risks of both methods (level of evidence: D (conflicting adult RCTs; no paediatric data)).
4.8 Conclusions and recommendations - prevention
Infection control remains the mainstay of VAP prevention. It is particularly important to emphasise this measure in resource-constrained PICUs with poor staffing levels. Although the 'bundle' approach reduces the incidence of VAP in adults,49 most components have not been validated in the paediatric age group, and many may not be suitable or practical for the PICU. In well-resourced countries with sufficient staffing, it may be appropriate to implement a number of low-risk interventions which may have some benefit. However, in South Africa, where resources are limited, unnecessary interventions should be avoided as these will increase the workload of overloaded nursing staff, and predispose to adverse events.87 Therefore, to avoid inappropriate use of scarce resources in an attempt to improve patient outcome, research is needed to evaluate all the 'bundle' interventions in the paediatric age group - including efficacy, potential harm, and optimal application.
By identifying effective preventive strategies appropriate to our population, the cost of this hospital-acquired infection could be reduced - a cost to the patient in terms of the physical and psychosocial effects of lengthy hospital stay, morbidity and mortality; and the financial cost to the patients' family and the state of lengthy PICU and hospital stays.
5. Treatment of VAP
Prior antibiotic therapy may select for resistant organisms already present in the respiratory tract, thereby predisposing to VAP.8 Prior use of carbapenems and third-generation cephalosporins are independent risk factors for acquisition of MDR A. baumannii.34 Therefore, all PICUs should enforce strict antibiotic restriction policies which specify indications for using carbapenems, cephalosporins, aminoglycosides, vancomycin and quinolones.
On clinical suspicion of VAP, patients should be cultured, preferably from the lower respiratory tract (e.g. by BAL), and empirical therapy changed or discontinued, based on these results and the clinical status.4 The culture results should be considered with other infectious markers such as PCT and band count, and these should be reviewed at 48 - 72 hours. If the cultures are negative and the PCT is low, one may consider stopping the empirical antibiotics unless there are other issues such as immunosuppression or low WCC. Fig. 1 provides an algorithm for the management of VAP, based on adult recommendations.88 We have modified the suggested broad-spectrum antibiotic treatment for suspected MDR organisms, mainly because most patients admitted to PICU in South Africa have significant risks for MDR pathogens. We are concerned that the use of cephalosporins or beta-lactam/ beta-lactamase inhibitors or carbapenems in combination with aminoglycosides or fluoroquinolones and vancomycin88 would predispose to more MDR organisms in our PICU settings. It is therefore suggested that unit-based policies should be developed according to the prevalent organisms in each PICU.

5.1 Principles of antimicrobial use in VAP
The principles determining antimicrobial use for VAP are best described in the statement: 'Choose empiric antimicrobial wisely (broad spectrum), start early, hit hard with an appropriate dosing schedule, de-escalate rapidly (narrow spectrum) and stop abruptly (post adequate duration)'. The most common cause of developing antimicrobial resistance is an inappropriately chosen antimicrobial at a sub-therapeutic dose for a long duration. Table III comprises a list of antimicrobials used commonly in PICUs, along with dosages, complications and other considerations.
Evidence that governs these practices is based on the following considerations:
5.1.1 Time of administration
Early administration of an antimicrobial regimen to which the pathogens are sensitive is one of the primary determinants of hospital outcome including treatment failure and death.89 The choice of empiric therapy should be carefully selected on the basis of specific disease state, resident pathogens within that environment and their associated resistance patterns, need for invasive procedures, recent hospitalisation, and the nutritional and immunological state of the patient.90 Given the increased likelihood of polymicrobial infections in view of the HIV epidemic in southern Africa, it would appear that broad-spectrum antimicrobial cover would be most appropriate.91 Initial empiric therapy should include a combination of agents.92 It has been recommended that empirical therapy for suspected VAP be started promptly, as delay is associated with higher mortality.93 However, empirical antibiotic treatment for suspected VAP accounts for a large proportion of inappropriate antibiotic usage in PICUs.94 Delay in introducing appropriate antibiotic therapy has been associated with adverse outcomes; therefore, antibiotics should be commenced promptly on clinical suspicion of VAP.95
5.1.2 Pharmacokinetic and pharmacodynamic principles
The pharmacodynamics and pharmacokinetics of the different medications in the recipient host must be established as best as possible. Disease states affect the body's ability to metabolise and excrete antimicrobial agents; patients with gut, hepatic or renal dysfunction need adjustments in the mode of drug delivery and dosage.96 The effects of the antimicrobials on the pathogens are governed by pharmacokinetic and pharmacodynamic principles including drug-drug interactions, and time- and concentration-dependent antimicrobial responses. Drug-drug interactions occur with various agents and require adjustments in terms of dosages or changes in the choice of antibiotics.97 The minimum inhibitory concentration (MIC) is the lowest concentration of an antibiotic that can inhibit the growth of the pathogen. The MIC90 is the minimum inhibitory concentration of the drug that is required to decrease growth of 90% of the pathogen. Not all isolates of a single pathogen have exactly the same MIC for a given antibiotic. The clinically relevant breakpoint is the level of the antimicrobial that should inhibit growth effectively. Failure to inhibit growth will determine the development of drug resistance.
Time-dependent killing and minimum persistent effects are based on the time when the antibiotic concentration is above the MIC.98 To be effective, the concentration of an antibiotic sharing these kinetics should be above the MIC for >40 - 50% of the dosing interval. These antibiotics must be dosed more frequently or a sustained- or extended-release formulation be used. In addition, the highest possible dose to ensure a drug level at least tenfold greater than the MIC is essential to ensure effective eradication of the pathogens and to prevent development of resistance. Penicillin, cephalosporin, linelozid, piperacillin-tazobactam and carbepenem are antibiotics that use time-dependent pharmacokinetic principles as a mode of action. Vancomycin uses a time-dependent killing and displays moderate to prolonged persistent effects and therefore requires the maximum amount of drug to be above the MIC for 24 hours. At present, carbepenem appears to have better time >MIC targets for K. pneumoniae and E. coli than third- or fourth-generation cephalosporin or piperacillin-tazobactam, owing to the lower levels of carbepenem resistance.99 Excessive use of these antibiotics is of concern, given that development of resistance is a class effect.100 Some antibiotics (e.g. clindamycin, vancomycin and linezolid) demonstrate a post-antibiotic effect that prevents re-growth of organisms within a specific period after antibiotic use.
Concentration-dependent killing and prolonged persistent effects are related either to the peak concentration above MIC or to the area under the Cmax curve (AUC) to the MIC ratio.101 The target (peak)- to-MIC ratio for optimal killing of Gram-negative enteric organisms as seen for aminoglycoside needs to be at least 10 times above the MIC to prevent organisms from developing resistance.102 AUC-to-MIC ratio targets for quinolones are >30 times that for bacterial eradication and >100 times that for preventing development of antimicrobial resistance; hence, quinolone resistance is common.103 AUC-to-MIC targets for quinolones are lower for Gram-positive than for Gram-negative pathogens. Adverse effects of aminoglycosides are related to high trough levels, and dosing schedules should aim at a single daily dose that would achieve high peaks and low trough. Inappropriate use of these agents will also result in the development of MDR non-fermenters.104
A particular problem of the PICU environment is that patients:
• are often fluid-overloaded or receiving high volumes of fluid for resuscitation from haemodynamic instability (which potentially decreases serum levels of water-soluble antibiotics)
• frequently have renal and/or hepatic dysfunction with delays in excretion of drugs (so conducing to potential toxicity)
• are being administered with several medications (so increasing drug interaction)
• have gut dysfunction (so having unpredictable uptake of drug from the gut)
• have rapid changes in serum protein levels (which may affect free drug levels profoundly).
Few antibiotics used in the PICU have been extensively studied in children, and dosage recommendations are frequently based on adult studies or extrapolated from children with less severe illnesses. Additional interventions, such as renal replacement therapy including dialysis or continuous haemofiltration, may affect drug levels. Therefore, measurement of drug levels with appropriate dosage adjustment is crucial to ensure adequate therapeutic drug levels.
5.1.3 De-escalation of antibiotic therapy
De-escalation of antibiotic therapy should occur as soon as a specific microbe is identified.105 Reversion to a narrow-spectrum antimicrobial in this situation will reduce the risk of removing commensals and will prevent resistance from developing. There is a clinical concern that it may be unwise to change a 'winning' antimicrobial regimen, but continuation of the broad-spectrum antimicrobial regimen has been associated with harm. Clinically relevant pathogens not covered by a narrow-spectrum antimicrobial are likely to be identified promptly if repeat microbiological screens are performed to ensure eradication of the primary pathogen or to pick up any missed secondary pathogen.
5.1.4 Cessation of therapy
Therapy should be stopped once an adequate duration of therapy has been completed. This is not easy to ensure. Many VAP patients acquire new non-microbial co-morbidities attributed to secondary nosocomial infections, which influences inappropriate prolonged use of antimicrobials. This risk is fuelled by the lack of knowledge of the exact duration of therapy for VAP. In general, antimicrobials should be used for a maximum of 7 days, or 3 days after there has been sufficient resolution as determined by clinical and laboratory markers.106 If there is lack of adequate response after 48 - 72 hours, patients should be re-screened for nosocomial pathogens. Drug-related factors such as plasma or tissue levels, protein binding, volume of distribution, drug-drug interactions, or host factors such as development of sanctuary sites of infection (e.g. abscesses, endocarditis, bone infection, indwelling catheters with phlebitis and recurrent infection e.g. aspiration pneumonia) should be considered.107, 108 Beta-lactam antibiotics have an oral bioavailability of only 5 - 10% compared with an intravenous dose, while quinolones and linezolid have excellent oral bioavailability with serum levels approximating intravenous doses. If there is no response by day 7, the antimicrobial should be stopped and the patient re-evaluated, with a revision in the diagnosis. There are exceptions to the general 7-day rule for some infections e.g. P. aeruginosa infections require 7 - 10 days of therapy, P. jiroveci 21 days, Gram-negative meningitis in newborns 14 - 21 days, tuberculosis 6 months, fungal infections 14 days, and staphylococcal empyemas 6 weeks of therapy.109, 110 In patients with MDR pathogens it is recommended that all antimicrobials should be withheld.6,111 Communication between the microbiologist and intensivist is essential to ensure the best possible outcomes.
Antibiotic resistance, defined according to current levels of clinically relevant breakpoints provided by the Centers for Disease Control in Atlanta, requires special mention. Resistance occurs through several mechanisms, viz. alteration of penicillin binding site, beta-lactamase and extended-spectrum beta-lactamase-producing organisms via chromosomal-inducible enzymes and cephalosporinase, ribosomal site alteration, efflux mediated mef E (high level) and target modifiable erm AM mediated (low level) resistance, alteration in the binding site of a specific transpeptidase (mec A) in methicillin-resistant S. aureus, point mutations in gyrase A,B in quinolone resistance, and para C,E or cell wall porin-protein deficiency associated with carbepenem resistance.112,113 Globally, there has been a substantial increase in the incidence of antibiotic resistance, more so in the private than public sectors. Intermediate-level resistance to penicillin by S. pneumoniae is between 25 and 40%, while macrolide resistance is >40%.114,115 The incidences of beta-lactamase-producing H. influenzae and M. catarrhalis are 6% and 80% respectively.116,117 High levels of ESBL and BSBL enterobacteriaceae (K. pneumoniae) (cephalosporin resistance of 26%), MDR Acinetobacter species (carbepenem resistance of 32%) and Pseudomonas species (42% carbepenem resistance) are seen in PICUs in South Africa.118,119 Increased resistance to aminoglycosides, pipercillin-tazobactam and fluoroquinolones has been recorded. Ertapenem, a first-generation carbepenem with poor efficacy against Pseudomonas, currently has low levels of resistance to ESBL-producing organisms, but there are concerns that with excessive use it is likely to induce cross-resistance to the entire carbepenem class of antimicrobials. Colistin and polymyxcin retain their sensitivity against MDR Gram-negative pathogens. Methicillin-resistant S. aureus has been isolated in approximately 15% of isolates from PICUs and is best treated with vancomycin, although linezolid is a useful alternative. In cases of vancomycin-resistant enterococcus, the use of newer staphylococcus drugs (e.g. quinupristin-dalfopristin) may be considered.
6. Conclusion
The evidence pertaining to paediatric VAP is scanty, with most recommendations extrapolated from adult studies from the developed world. This may not be appropriate because of anatomical and physiological differences between adults and children. Very little information is available relating to VAP in South Africa. Considering that our paediatric population is fundamentally different from that of developed countries, it is essential that clinical studies be conducted in our population, to develop evidence-based guidelines for the prevention and treatment of paediatric VAP in this country.
This guideline has been endorsed by the Critical Care Society of South Africa, and its publication sponsored by an unrestricted education grant from Pfizer Pharmaceuticals. Dr Morrow was supported by grants from the Medical Research Council of Southern Africa and the University of Cape Town.
7. References
1. Elward AM. Pediatric ventilator-associated pneumonia. Pediatr Infect Dis J 2003; 22: 445-446. [ Links ]
2. Corley DE, Kirtland SH, Winterbauer RH, et al. Reproducibility of the histologic diagnosis of pneumonia among a panel of four pathologists: Analysis of a gold standard. Chest 1997; 112: 458-465. [ Links ]
3. Kirtland SH, Corley DE, Winterbauer RH, et al. The diagnosis of ventilator-associated pneumonia: A comparison of histologic, microbiologic, and clinical criteria. Chest 1997; 112: 445-457. [ Links ]
4. Foglia E, Meier MD, Elward A. Ventilator-associated pneumonia in neonatal and pediatric intensive care unit patients. Clin Microbiol Rev 2007; 20: 409-425. [ Links ]
5. Morrow BM, Argent AC. Ventilator-associated pneumonia in a paediatric intensive care unit in a developing country with high HIV-prevalence. A retrospective survey. J Paediatr Child Health; In press. [ Links ]
6. Jeena P, Thompson E, Nchabeleng M, Sturm A. Emergence of multi-drug-resistant acinetobacter anitratus species in neonatal and paediatric intensive care units in a developing country: Concern about antimicrobial policies. Ann Trop Paediatr 2001; 21: 245-251. [ Links ]
7. Elward AM, Warren DK, Fraser VJ. Ventilator-associated pneumonia in pediatric intensive care unit patients: Risk factors and outcomes. Pediatrics 2002; 109: 758-764. [ Links ]
8. Almuneef M, Memish ZA, Balkhy HH, Alalem H, Abutaleb A. Ventilator-associated pneumonia in a pediatric intensive care unit in Saudi Arabia: A 30-month prospective surveillance. Infect Control Hosp Epidemiol 2004; 25: 753-778. [ Links ]
9. Cummins P. Access to health care in the Western Cape. Lancet 2002; 360: s49-50. [ Links ]
10. Miller MR, Gergen P, Honour M, Zhan C. Burden of illness for children and where we stand in measuring the quality of this health care. Ambul Pediatr 2005; 5: 268-278. [ Links ]
11. Bradshaw D, Nannan N, Laubscher R, et al. Mortality Estimates for Western Cape Province, 2000. South African National Burden of Disease Study. Medical Research Council of South Africa; 2005. [ Links ]
12. Arabi Y, Al-Shirawi N, Memish Z, Anzueto A. Ventilator-associated pneumonia in adults in developing countries: A systematic review. Int J Infect Dis 2008; 12: 505-512. [ Links ]
13. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008; 36: 309-332. [ Links ]
14. Pieracci FM, Barie PS. Strategies in the prevention and management of ventilator-associated pneumonia. Am Surg 2007; 73: 419-432. [ Links ]
15. Schurink CA, Van Nieuwenhoven CA, Jacobs JA, et al. Clinical pulmonary infection score for ventilator-associated pneumonia: Accuracy and inter-observer variability. Intensive Care Med 2004 ; 30: 217-224. [ Links ]
16. Wright ML, Romano MJ. Ventilator-associated pneumonia in children. Semin Pediatr Infect Dis 2006; 17: 58-64. [ Links ]
17. Price MB, Grant MJ, Welkie K. Financial impact of elimination of routine chest radiographs in a pediatric intensive care unit. Crit Care Med 1999; 27: 1588-1593. [ Links ]
18. Soboleski D, Theriault C, Acker A, Dagnone V, Manson D. Unnecessary irradiation to nonthoracic structures during pediatric chest radiography. Pediatr Radiol 2006; 36: 22-25. [ Links ]
19. Davies HD, Wang EE, Manson D, Babyn P, Shuckett B. Reliability of the chest radiograph in the diagnosis of lower respiratory infections in young children. Pediatr Infect Dis J 1996; 15: 600-604. [ Links ]
20. Meade MO, Cook RJ, Guyatt GH, et al. Interobserver variation in interpreting chest radiographs for the diagnosis of acute respiratory distress syndrome. Am J Respir Crit Care Med 2000; 161: 85-90. [ Links ]
21. Graat ME, Stoker J, Vroom MB, Schultz MJ. Can we abandon daily routine chest radiography in intensive care patients? J Intensive Care Med 2005; 20: 238-246. [ Links ]
22. El-Radhi AS, Barry W. Thermometry in paediatric practice. Arch Dis Child 2006; 91: 351-356. [ Links ]
23. Goldstein B, Giroir B, Randolph A, International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 2005; 6: 2-8. [ Links ]
24. van Rossum AM, Wulkan RW, Oudesluys-Murphy AM. Procalcitonin as an early marker of infection in neonates and children. Lancet Infect Dis 2004; 4: 620-630. [ Links ]
25. Rubin BK. Designing clinical trials to evaluate mucus clearance therapy. Respir Care 2007; 52: 1348-1358. [ Links ]
26. Elphick HE, Lancaster GA, Solis A, Majumdar A, Gupta R, Smyth RL. Validity and reliability of acoustic analysis of respiratory sounds in infants. Arch Dis Child 2004; 89: 1059-1063. [ Links ]
27. Alsmadi S, Kahya YP. Design of a DSP-based instrument for real-time classification of pulmonary sounds. Comput Biol Med 2008; 38: 53-61. [ Links ]
28. Gauvin F, Dassa C, Chaibou M, Proulx F, Farrell CA, Lacroix J. Ventilator-associated pneumonia in intubated children: Comparison of different diagnostic methods. Pediatr Crit Care Med 2003; 4: 437-443. [ Links ]
29. Fagon JY, Chastre J. Diagnosis and treatment of nosocomial pneumonia in ALI/ARDS patients. Eur Respir J Suppl 2003; 42: 77s-83s. [ Links ]
30. Grigg J, Van den Borre C, Malfroot A, Pierard D, Wang D, Dab I. Bilateral fiberoptic bronchoalveolar lavage in acute unilateral lobar pneumonia. J Pediatr 1993; 122: 606-608. [ Links ]
31. Morrow B, Argent A. Risks and complications of nonbronchoscopic bronchoalveolar lavage in a pediatric intensive care unit. Pediatr Pulmonol 2001; 32: 378-384. [ Links ]
32. Morrow B, Futter M, Argent A. A simple method of reducing complications of pediatric nonbronchoscopic bronchoalveolar lavage. Pediatr Pulmonol 2004; 38: 217-221. [ Links ]
33. Fayon MJ, Tucci M, Lacroix J, et al. Nosocomial pneumonia and tracheitis in a pediatric intensive care unit: A prospective study. Am J Respir Crit Care Med 1997; 155: 162-169. [ Links ]
34. Falagas ME, Kopterides P. Risk factors for the isolation of multi-drug-resistant acinetobacter baumannii and pseudomonas aeruginosa: A systematic review of the literature. J Hosp Infect 2006; 64: 7-15. [ Links ]
35. Abramczyk ML, Carvalho WB, Carvalho ES, Medeiros EA. Nosocomial infection in a pediatric intensive care unit in a developing country. Braz J Infect Dis 2003; 7: 375-380. [ Links ]
36. Tullu MS, Deshmukh CT, Baveja SM. Bacterial nosocomial pneumonia in paediatric intensive care unit. J Postgrad Med 2000; 46: 18-22. [ Links ]
37. Principi N, Esposito S. Ventilator-associated pneumonia (VAP) in pediatric intensive care units. Pediatr Infect Dis J 2007; 26: 841-844. [ Links ]
38. Madhi SA, Ismail K, O'Reilly C, Cutland C. Importance of nosocomial respiratory syncytial virus infections in an African setting. Trop Med Int Health 2004; 9: 491-498. [ Links ]
39. Hatherill M, Levin M, Lawrenson J, Hsiao NY, Reynolds L, Argent A. Evolution of an adenovirus outbreak in a multidisciplinary children's hospital. J Paediatr Child Health 2004; 40: 449-454. [ Links ]
40. Wesley AG, Pather M, Tait D. Nosocomial adenovirus infection in a paediatric respiratory unit. J Hosp Infect 1993; 25: 183-190. [ Links ]
41. Iten A, Chave JP, Wauters JP, Maziero A, Francioli P. Pneumocystis carinii pneumonia (PCP) in HIV negative patients (PTS): Nosocomial transmission? Int Conf Aids 2003; 9: 379. [ Links ]
42. Petrosillo N, Nicastri E, Viale P. Nosocomial pulmonary infections in HIV-positive patients. Curr Opin Pulm Med 2005; 11: 231-235. [ Links ]
43. Stein F, Trevino R. Nosocomial infections in the pediatric intensive care unit. Pediatr Clin North Am 1994; 41: 1245-1257. [ Links ]
44. Podnos YD, Cinat ME, Wilson SE, Cooke J, Gornick W, Thrupp LD. Eradication of multi-drug resistant Acinetobacter from an intensive care unit. Surg Infect (Larchmt) 2001; 2: 297-301. [ Links ]
45. Crnich CJ, Safdar N, Maki DG. The role of the intensive care unit environment in the pathogenesis and prevention of ventilator-associated pneumonia. Respir Care 2005; 50: 813-836. [ Links ]
46. Hugonnet S, Uckay I, Pittet D. Staffing level: A determinant of late-onset ventilator-associated pneumonia. Crit Care 2007; 11: R80. [ Links ]
47. Stone PW, Mooney-Kane C, Larson EL, et al. Nurse working conditions and patient safety outcomes. Med Care 2007; 45: 571-578. [ Links ]
48. Hugonnet S, Chevrolet JC, Pittet D. The effect of workload on infection risk in critically ill patients. Crit Care Med 2007; 35: 76-81. [ Links ]
49. Curley MA, Schwalenstocker E, Deshpande JK, et al. Tailoring the Institute for Health Care Improvement 100,000 lives campaign to pediatric settings: The example of ventilator-associated pneumonia. Pediatr Clin North Am 2006; 53: 1231-1251. [ Links ]
50. Tablan OC, Anderson LJ, Besser R, Bridges C, Hajjeh R, CDC, Healthcare Infection Control Practices Advisory Committee. Guidelines for preventing health-care--associated pneumonia, 2003: Recommendations of CDC and the healthcare infection control practices advisory committee. MMWR Recomm Rep 2004; 53: 1-36. [ Links ]
51. Drakulovic MB, Torres A, Bauer TT, Nicolas JM, Nogue S, Ferrer M. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: A randomised trial. Lancet 1999; 354: 1851-1858. [ Links ]
52. Orozco-Levi M, Torres A, Ferrer M, et al. Semirecumbent position protects from pulmonary aspiration but not completely from gastroesophageal reflux in mechanically ventilated patients. Am J Respir Crit Care Med 1995; 152: 1387-1390. [ Links ]
53. Torres A, Serra-Batlles J, Ros E, et al. Pulmonary aspiration of gastric contents in patients receiving mechanical ventilation: The effect of body position. Ann Intern Med 1992; 116: 540-543. [ Links ]
54. Kallet RH, Quinn TE. The gastrointestinal tract and ventilator-associated pneumonia. Respir Care 2005; 50: 910-921. [ Links ]
55. Kress JP, Pohlman AS, O'Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med 2000; 342: 1471-1474. [ Links ]
56. Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (awakening and breathing controlled trial): A randomised controlled trial. Lancet 2008; 371: 126-134. [ Links ]
57. Little LA, Koenig JC Jr, Newth CJ. Factors affecting accidental extubations in neonatal and pediatric intensive care patients. Crit Care Med 1990; 18: 163-165. [ Links ]
58. Twite MD, Friesen RH. Pediatric sedation outside the operating room: The year in review. Curr Opin Anaesthesiol 2005; 18: 442-446. [ Links ]
59. Courtman SP, Wardurgh A, Petros AJ. Comparison of the bispectral index monitor with the comfort score in assessing level of sedation of critically ill children. Intensive Care Med 2003; 29: 2239-2246. [ Links ]
60. Crain N, Slonim A, Pollack MM. Assessing sedation in the pediatric intensive care unit by using BIS and the COMFORT scale. Pediatr Crit Care Med 2002; 3: 11-14. [ Links ]
61. Randolph AG, Wypij D, Venkataraman ST, et al. Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network. Effect of mechanical ventilator weaning protocols on respiratory outcomes in infants and children: A randomized controlled trial. JAMA 2002; 288: 2561-2568. [ Links ]
62. Cook DJ, Reeve BK, Guyatt GH, et al. Stress ulcer prophylaxis in critically ill patients. Resolving discordant meta-analyses. JAMA 1996; 275: 308-314. [ Links ]
63. Lopriore E, Markhorst DG, Gemke RJ. Ventilator-associated pneumonia and upper airway colonisation with gram negative bacilli: The role of stress ulcer prophylaxis in children. Intensive Care Med 2002; 28: 763-777. [ Links ]
64. Yildizdas D, Yapicioglu H, Yilmaz HL. Occurrence of ventilator-associated pneumonia in mechanically ventilated pediatric intensive care patients during stress ulcer prophylaxis with sucralfate, ranitidine, and omeprazole. J Crit Care 2002; 17: 240-245. [ Links ]
65. Wright ML, Romano MJ. Ventilator-associated pneumonia in children. Semin Pediatr Infect Dis 2006; 17: 58-64. [ Links ]
66. Cobley M, Atkins M, Jones PL. Environmental contamination during tracheal suction. A comparison of disposable conventional catheters with a multiple-use closed system device. Anaesthesia 1991; 46: 957-961. [ Links ]
67. Freytag CC, Thies FL, Konig W, Welte T. Prolonged application of closed in-line suction catheters increases microbial colonization of the lower respiratory tract and bacterial growth on catheter surface. Infection 2003; 31: 31-37. [ Links ]
68. Jongerden IP, Rovers MM, Grypdonck MH, Bonten MJ. Open and closed endotracheal suction systems in mechanically ventilated intensive care patients: A meta-analysis. Crit Care Med 2007; 35: 260-270. [ Links ]
69. Lasocki S, Lu Q, Sartorius A, Fouillat D, Remerand F, Rouby JJ. Open and closed-circuit endotracheal suctioning in acute lung injury: Efficiency and effects on gas exchange. Anesthesiology 2006; 104: 39-47. [ Links ]
70. Lindgren S, Almgren B, Hogman M. Effectiveness and side effects of closed and open suctioning: An experimental evaluation. Intensive Care Med 2004; 30: 1630-1637. [ Links ]
71. Peter JV, Chacko B, Moran JL. Comparison of closed endotracheal suction versus open endotracheal suction in the development of ventilator-associated pneumonia in intensive care patients: An evaluation using meta-analytic techniques. Indian J Med Sci 2007; 61: 201-211. [ Links ]
72. Vonberg RP, Eckmanns T, Welte T, Gastmeier P. Impact of the suctioning system (open vs. closed) on the incidence of ventilation-associated pneumonia: Meta-analysis of randomized controlled trials. Intensive Care Med 2006; 32: 1329-1335. [ Links ]
73. Cordero L, Sananes M, Ayers LW. Comparison of a closed (trach care MAC) with an open endotracheal suction system in small premature infants. J Perinatol 2000; 20: 151-156. [ Links ]
74. Munro CL, Grap MJ. Oral health and care in the intensive care unit: State of the science. Am J Crit Care 2004; 13: 25-33. [ Links ]
75. Fitch JA, Munro CL, Glass CA, Pellegrini JM. Oral care in the adult intensive care unit. Am J Crit Care 1999; 8: 314-318. [ Links ]
76. Koeman M, van der Ven AJ, Hak E, et al. Oral decontamination with chlorhexidine reduces the incidence of ventilator-associated pneumonia. Am J Respir Crit Care Med 2006; 173: 1348-1355. [ Links ]
77. Kononen E. Anaerobes in the upper respiratory tract in infancy. Anaerobe 2005; 11: 131-136. [ Links ]
78. Holzapfel L, Chastang C, Demingeon G, Bohe J, Piralla B, Coupry A. A randomized study assessing the systematic search for maxillary sinusitis in nasotracheally mechanically ventilated patients. Influence of nosocomial maxillary sinusitis on the occurrence of ventilator-associated pneumonia. Am J Respir Crit Care Med 1999; 159: 695-701. [ Links ]
79. Bach A, Boehrer H, Schmidt H, Geiss HK. Nosocomial sinusitis in ventilated patients. nasotracheal versus orotracheal intubation. Anaesthesia 1992; 47: 335-339. [ Links ]
80. Frank JA, Parsons PE, Matthay MA. Pathogenetic significance of biological markers of ventilator-associated lung injury in experimental and clinical studies. Chest 2006; 130: 1906-1914. [ Links ]
81. Holzapfel L, Chevret S, Madinier G, et al. Influence of long-term oro- or nasotracheal intubation on nosocomial maxillary sinusitis and pneumonia: Results of a prospective, randomized, clinical trial. Crit Care Med 1993; 21: 1132-1138. [ Links ]
82. Stein M, Caplan ES. Nosocomial sinusitis: A unique subset of sinusitis. Curr Opin Infect Dis 2005; 18: 147-150. [ Links ]
83. George DL, Falk PS, Umberto Meduri G, et al. Nosocomial sinusitis in patients in the medical intensive care unit: A prospective epidemiological study. Clin Infect Dis 1998; 27: 463-470. [ Links ]
84. van Zanten AR, Tjan DH, Polderman KH. Preventing nosocomial sinusitis in the ICU: Comment on article by Pneumatikos et al. Intensive Care Med 2006; 32: 1451. [ Links ]
85. Bottei K. Feeding dysfunction: A nursing diagnosis for infants who resist oral feeding. Nurs Diagn 1995; 6: 80-88. [ Links ]
86. Amantea SL, Piva JP, Sanches PR, Palombini BC. Oropharyngeal aspiration in pediatric patients with endotracheal intubation. Pediatr Crit Care Med 2004; 5: 152-156. [ Links ]
87. Frey B, Argent A. Safe paediatric intensive care. Part 1: Does more medical care lead to improved outcome? Intensive Care Med 2004; 30: 1041-1046. [ Links ]
88. Porzecanski I, Bowton DL. Diagnosis and treatment of ventilator-associated pneumonia. Chest 2006; 130: 597-604. [ Links ]
89. Khardori N. Antibiotics - past, present, and future. Med Clin North Am 2006; 90: 1049-1076. [ Links ]
90. Beardsley JR, Williamson JC, Johnson JW, Ohl CA, Karchmer TB, Bowton DL. Using local microbiologic data to develop institution-specific guidelines for the treatment of hospital-acquired pneumonia. Chest 2006; 130: 787-793. [ Links ]
91. Brink A, Moolman J, da Silva MC, Botha M National Antibiotic Surveillance Forum. Antimicrobial susceptibility profile of selected bacteraemic pathogens from private institutions in South Africa. S Afr Med J 2007; 97: 273-279. [ Links ]
92. Hanberger H, Arman D, Gill H, et al. Surveillance of microbial resistance in European intensive care units: A first report from the Care-ICU programme for improved infection control. Intensive Care Med 2008. Epub ahead of print. [ Links ]
93. Iregui M, Ward S, Sherman G, Fraser VJ, Kollef MH. Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator-associated pneumonia. Chest 2002; 122: 262-268. [ Links ]
94. Fischer JE, Ramser M, Fanconi S. Use of antibiotics in pediatric intensive care and potential savings. Intensive Care Med 2000; 26: 959-966. [ Links ]
95. Burkhardt O, Hafer C, Langhoff A, et al. Pharmacokinetics of ertapenem in critically ill patients with acute renal failure undergoing extended daily dialysis. Nephrol Dial Transplant 2008. Epub ahead of print. [ Links ]
96. Thomson KS, Moland ES. Cefepime, piperacillin-tazobactam, and the inoculum effect in tests with extended-spectrum beta-lactamase-producing enterobacteriaceae. Antimicrob Agents Chemother 2001; 45: 3548-3554. [ Links ]
97. Viswanatha T, Marrone L, Goodfellow V, Dmitrienko GI. Assays for beta-lactamase activity and inhibition. Methods Mol Med 2008; 142: 239-260. [ Links ]
98. Kaye CM, Allen A, Perry S, et al. The clinical pharmacokinetics of a new pharmacokinetically enhanced formulation of amoxicillin/clavulanate. Clin Ther 2001; 23: 578-584. [ Links ]
99. Raveh D, Muallem-Zilcha E, Greenberg A, Wiener-Well Y, Schlesinger Y, Yinnon AM. Prospective drug utilization evaluation of three broad-spectrum antimicrobials: Cefepime, piperacillin-tazobactam and meropenem. QJM 2006; 99: 397-406. [ Links ]
100. Bradley JS, Guidos R, Baragona S, et al. Anti-infective research and development--problems, challenges, and solutions. Lancet Infect Dis 2007; 7: 68-78. [ Links ]
101. Zhanel GG. Influence of pharmacokinetic and pharmacodynamic principles on antibiotic selection. Curr Infect Dis Rep 2001; 3: 29-34. [ Links ]
102. Mueller M, de la Peña A, Derendorf H. Issues in Pharmacokinetics and Pharmacodynamics of Anti-Infective Agents: Kill Curves versus MIC. Antimicrob Agents Chemother 2004; 48(2): 369-377. [ Links ]
103. Chen DK, McGeer A, de Azavedo JC, Low DE. Decreased susceptibility of streptococcus pneumoniae to fluoroquinolones in Canada. Canadian bacterial surveillance network. N Engl J Med 1999; 341: 233-239. [ Links ]
104. Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med 2002; 165: 867-903. [ Links ]
105. American Thoracic Society, Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005; 171: 388-416. [ Links ]
106. Goldberg J, Owens RC. Optimizing antimicrobial dosing in the critically ill patient. Curr Opin Crit Care 2002; 8: 435-440. [ Links ]
107. Kollef MH, Shorr A, Tabak YP, Gupta V, Liu LZ, Johannes RS. Epidemiology and outcomes of health-care-associated pneumonia: Results from a large US database of culture-positive pneumonia. Chest 2005; 128: 3854-3862. [ Links ]
108. Shorr AF, Sherner JH, Jackson WL, Kollef MH. Invasive approaches to the diagnosis of ventilator-associated pneumonia: A meta-analysis. Crit Care Med 2005; 33: 46-53. [ Links ]
109. Gaynes R, Edwards JR, National Nosocomial Infections Surveillance System. Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis 2005; 41: 848-854. [ Links ]
110. Ibrahim EH, Ward S, Sherman G, Schaiff R, Fraser VJ, Kollef MH. Experience with a clinical guideline for the treatment of ventilator-associated pneumonia. Crit Care Med 2001; 29: 1109-1115. [ Links ]
111. Lorente L, Blot S, Rello J. Evidence on measures for the prevention of ventilator-associated pneumonia. Eur Respir J 2007; 30: 1193-1207. [ Links ]
112. Heffelfinger JD, Dowell SF, Jorgensen JH, et al. Management of community-acquired pneumonia in the era of pneumococcal resistance: A report from the drug-resistant streptococcus pneumoniae therapeutic working group. Arch Intern Med 2000; 160: 1399-1408. [ Links ]
113. Hyde TB, Gay K, Stephens DS, et al. Macrolide resistance among invasive streptococcus pneumoniae isolates. JAMA 2001; 286: 1857-1862. [ Links ]
114. Doern GV, Heilmann KP, Huynh HK, Rhomberg PR, Coffman SL, Brueggemann AB. Antimicrobial resistance among clinical isolates of streptococcus pneumoniae in the United States during 1999-2000, including a comparison of resistance rates since 1994-1995. Antimicrob Agents Chemother 2001; 45: 1721-1729. [ Links ]
115. Hoban DJ, Wierzbowski AK, Nichol K, Zhanel GG. Macrolide-resistant streptococcus pneumoniae in Canada during 1998-1999: Prevalence of mef(A) and erm(B) and susceptibilities to ketolides. Antimicrob Agents Chemother 2001; 45: 2147-2150. [ Links ]
116. Zhanel GG, DeCorby M, Laing N, et al. Antimicrobial-resistant pathogens in intensive care units in Canada: Results of the Canadian National Intensive Care Unit (CAN-ICU) study, 2005-2006. Antimicrob Agents Chemother 2008; 52: 1430-1437. [ Links ]
117. Petros AJ, O'Connell M, Roberts C, Wade P, van Saene HK. Systemic antibiotics fail to clear multidrug-resistant Klebsiella from a pediatric ICU. Chest 2001; 119: 862-866. [ Links ]
118. Felmingham D, Washington J. Trends in the antimicrobial susceptibility of bacterial respiratory tract pathogens--findings of the Alexander Project 1992-1996. J Chemother 1999; 11: Suppl 1: 5-21. [ Links ]
119. Jacobs MR, Felmingham D, Appelbaum PC, Gruneberg RN, The Alexander Project Group. The Alexander Project 1998-2000: Susceptibility of pathogens isolated from community-acquired respiratory tract infection to commonly used antimicrobial agents. J Antimicrob Chemother 2003; 52: 229-246. [ Links ]
120. Cunha BA, ed. Antibiotic Essentials. 5th ed. Royal Oak, Mich., USA: Physician's Press, 2006. [ Links ]
 Correspondence to:
Correspondence to:
Professor R Green
Department of Paediatrics and Child Health
University of Pretoria, PO Box 667, Pretoria 0001
tel. 012 354-5272, fax 012 354-5275
email robin.green@up.ac.za














