Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
SAMJ: South African Medical Journal
versión On-line ISSN 2078-5135
versión impresa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.99 no.4 Pretoria abr. 2009
ORIGINAL ARTICLES
The incidence and clinical presentation of infantile rotavirus diarrhoea in Sierra Leone
François P R de VilliersI; Thomas N SawyerrII; Gillian K de VilliersIII
IMMed, FACP, PhD. Department of Paediatrics and Child Health, Medunsa Campus, University of Limpopo (Medunsa Campus), Pretoria
IIMB ChB, DCH, MSc. Department of Paediatrics and Child Health, University of Limpopo (Medunsa Campus), and Ola During Children's Hospital, Freetown, Sierra Leone
IIIMSc. Department of Paediatrics and Child Health and MRC/Medunsa Diarrhoeal Pathogens Unit, University of Limpopo (Medunsa Campus)
ABSTRACT
OBJECTIVE: An effective vaccine is needed to protect against severe rotavirus disease, an important cause of gastroenteritis. Since there are no data on the incidence and antigenic diversity of rotavirus infection in Sierra Leone, we studied its epidemiology to enable an effective vaccine strategy to be designed.
METHODS: Children between the ages of 3 and 30 months presenting with gastroenteritis to the Ola During Children's Hospital in Freetown, Sierra Leone, were enrolled. Stool specimens were tested in South Africa using polyacrylamide gel electrophoresis to confirm rotavirus infection.
RESULTS: Over a 5-month period 143 children presenting with gastroenteritis were recruited. Stool samples obtained from 128 study subjects were tested for the presence of rotavirus; 45% were aged between 3 and 9 months (mean age 10.85 months), and 48 stool samples (37.5%) tested positive for rotavirus. The incidence of rotavirus infection was 20% higher in boys than in girls, a gender difference confirmed elsewhere in West Africa. The prevalence of rotavirus-positive stools peaked in August, coinciding with the rainy season. About 90% of the rotavirus-positive patients had severe diarrhoea, as opposed to only about two-thirds of the patients whose diarrhoea was not caused by rotavirus; this difference was statistically significant.
CONCLUSIONS: There is a high incidence of rotavirus infection in Sierra Leone, with rotavirus causing 37.5% of the gastroenteritis in this study. Patients with rotavirus gastroenteritis almost all had severe diarrhoea. The high incidence of rotavirus infection and the severity of the disease presentation make the institution of a rotavirus vaccine programme in Sierra Leone imperative.
Rotaviruses occur widely among humans and many animal species throughout the world, and are important causes of gastroenteritis. Since their discovery over 30 years ago human rotaviruses have been shown to be the major cause of paediatric diarrhoeal disease morbidity and mortality.1
The virus will have infected some 90% of children by the age of 3 and practically all children by the age of 5,2 and accounts for approximately 600 000 deaths worldwide every year.3 The incidence of rotavirus disease in children is similar in developed and developing countries, but mortality from diarrhoeal disease is higher in the latter, predominantly owing to poor knowledge of oral rehydration therapy and malnutrition. In Africa rotavirus accounts for about a quarter of hospitalisations of children for diarrhoea.4,5 A study from 15 African countries done to assess epidemiology and disease burden suggests that rotavirus is the most important cause of severe diarrhoea in African children.4 Public health measures to provide clean water and improved hygiene and sanitation may affect the incidence of diarrhoea caused by bacterial infection, but not that of rotavirus.4 Vaccines have been shown to be the only means of reducing morbidity and mortality due to rotavirus.1,5,6 In industrialised countries with good standards of hygiene and clean food and water rotavirus remains the most common cause of diarrhoea-associated hospitalisations among young children.3 There is an urgent need to develop an effective vaccine to protect against severe rotavirus disease, and data on the incidence of rotavirus infections in Africa and the antigenic and genomic diversity of the rotavirus are required for the design of an effective vaccine strategy.
Since no rotavirus incidence studies have been done in Sierra Leone, this study was designed to determine the epidemiology of rotavirus infections in that country to inform policy makers on the need for the introduction of new rotavirus vaccines.
Methodology
This descriptive study of children between the ages of 3 and 30 months admitted with gastroenteritis was conducted at the Ola During Children's Hospital, the main paediatric referral hospital in Sierra Leone.
The target enrolment was 40 cases per month for a period of 5 months during the diarrhoea season. Children with dysentery (bloody diarrhoea or bloodstained stools) were excluded. On admission the relevant demographic data and history with regard to onset, duration and severity of the presenting complaints were obtained from the parents. Clinical data, including anthropometric measurements, were recorded on a data sheet.
Specimens were obtained by collecting stools from each study subject and were screened in Sierra Leone (by TNS) for the presence of rotavirus antigen using Rota-Strip (CORIS BioConcept), an immunochromatographic test for rotavirus detection in stool specimens. The samples were also tested in the laboratory in South Africa (by GKdeV) using polyacrylamide gel electrophoresis (PAGE) to confirm rotavirus infection and to screen for non-A rotaviruses. The PAGE gel results were used during the data analysis unless the results were indeterminate, when the CORIS results were used.
A pilot study of 15 cases conducted at Medunsa and 5 at the Children's Hospital in Freetown, done to validate the methodology and clinical data sheet, was not included in the study results.
Informed written consent was obtained from the parent or guardian of each child in the study. Approval for the study was obtained from the Research, Ethics and Publication Committee of the Faculty of Medicine of Medunsa and the Ethics Committee of the Sierra Leone Medical School, Freetown.
Data were entered in duplicate and analysed in Epi Info 2000. Descriptive statistics were performed. Chi-square tests were done to test for association between categorical variables.
Results
Over a 5-month period 143 children were enrolled. The initial enrolment target of 200 cases was not fulfilled because a strike by hospital staff precluded recruitment, and a number of gastroenteritis patients were sent home before enrolment. The age and gender distributions of the 128 subjects in whom stool samples were obtained for rotavirus testing are set out in Table I. There were more males (55.5%) than females, and 45.3% of the patients were aged between 3 and 9 months (mean age and median age were both 10.85 months) (standard deviation (SD) 5.68).
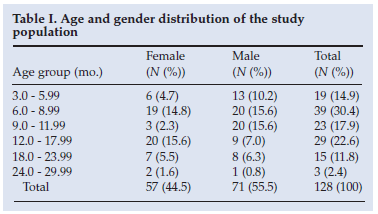
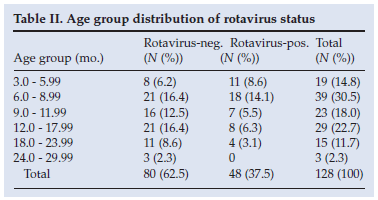
Of the 128 stool samples 48 (37.5%) tested positive for rotavirus; 31 (64.6%) of the rotavirus-positive cases were males and 17 (35.4%) were females, but the difference was not statistically significant (p=0.1094). Of the patients with rotavirus-negative results 39 (48.8%) were male. Table II presents the relative distribution of rotavirus infection among the age groups in the study. The median age for rotavirus infection was 8.25 months (mean 9.15 months; SD 4.81 months). There was an excessive number of cases of rotavirus infection in the two youngest age groups (3 - 9 months), in which 60% of the infections occurred; 36 (75.0%) of the rotavirus-positive children were younger than 12 months and 44 (91.6%) were younger than 18 months, while 66 (83.0%) of the control cases fell into the latter age group. The observed difference in the incidence of rotavirus infection between children younger than 1 year and those older than 1 year was statistically significant (p=0.0338). No patient over the age of 24 months tested positive for rotavirus.
During July the prevalence of rotavirus-positive stools was 52.6% (10 of 19 tests positive), in August it was 64.3% (18 of 28), in September it was 53.3% (8 of 15), in October it was 21.2% (7 of 33), in November it was 18.5% (5 of 27) and in December it was 0% (0 of 6). These differences were statistically significant (p=0.0007).
The rotavirus-positive patients all presented with diarrhoea, vomiting and fever, while most also had a cough or a cold. There was no significant difference between the two groups with regard to presenting symptoms or nutritional status (p=0.90 and 0.48). According to the Wellcome Trust classification of protein energy malnutrition, 77 patients (60.1%) were of normal weight for age, 40 (31.2%) were underweight for age, 4 (3.1%) had kwashiorkor, 5 (3.9%) were marasmic and 2 (1.6%) had marasmic kwashiorkor.
Disease severity was based on a 20-point Vesikari score differentiating mild, moderate and severe gastroenteritis; 43 (89.6%) of the rotavirus-positive cases had scores of 11 and above (indicative of severe diarrhoea) compared with 52 (65%) of rotavirus-negative cases. This difference was statistically significant (p=0.007), and is a major finding of this study. More than 80% of the subjects had some dehydration, but severe dehydration was uncommon. There were no differences between the groups.
A large number of clinical signs were elicited in both groups. Fever was present in 29% of rotavirus-negative versus 48% of rotavirus-positive subjects, oral thrush in 14% v. 21%, splenomegaly in 6% v. 21% and hepatomegaly in 21% v. 31%. In the RV-negative group, severe respiratory symptoms such as respiratory distress (9% v. 4%), and crepitations (19% v. 6%) were commoner than in the study group, while symptoms of cough occurred in about two-thirds of all subjects and sore throat (18% v. 7%) was commoner in the rotavirus-positive group. Cardiovascular and central nervous system symptoms were uncommon in both groups. Over 80% of subjects in both groups had general signs and symptoms of illness; approximately two-thirds had respiratory signs and one-third gastrointestinal signs (excluding diarrhoea and vomiting). Generally rotavirus-positive patients had more systemic signs and symptoms than rotavirus-negative patients, but this was not statistically significant. With regard to respiratory infections, upper respiratory tract infection (rhinitis, otitis media, tonsillitis, pharyngitis) was most common in both groups, with 21 cases (43.8%) in the rotavirus-positive group and 26 (32.5%) in the rotavirus-negative group. Pneumonia was diagnosed in less than a fifth of patients in both groups.
The most common co-morbidities (together with gastroenteritis) diagnosed by the admitting physicians were malaria, the most common diagnosis in both groups (nearly 90%), followed by respiratory tract infection (RTI). RTI was more common in the rotavirus-positive group (66.7% of cases) than in the rotavirus-negative group (41.3%). Other conditions, found in 10 patients, were typhoid fever, otitis media, anaemia, dermatological conditions (e.g. scabies, napkin dermatitis) and anaemia. Malnutrition was diagnosed by doctors in only 4 cases. This contrasts with nutritional status based on the Wellcome Classification, for which figures were given above. The differences were not statistically significant
Discussion
The role of rotavirus as an important cause of severe diarrhoeal disease in young children worldwide is well established. However, there is much to be learnt about the epidemiology, clinical features and differences in the disease profiles between different regions of the world.
Since no previous rotavirus studies have been done in Sierra Leone, our finding of an incidence of 37.5% is unique. Incidences found in other West African countries were 33% in Jos, Nigeria (with a higher incidence (45%) in children under the age of 6 months),7 27% in Zaria, Northern Nigeria (again with an incidence of 45% in those under 6 months),8 39% in Ghana and 36% in The Gambia.9 Incidence data appear to be similar for countries in this region. The consistency of these data over time and the different studies may help other countries in the region that do not have data to apply to the Global Alliance for Vaccines and Immunization for funding for rotavirus vaccines.
The demography of the study sample corresponds with the age at which the incidence of rotavirus infections is highest.4 Rotavirus is the cause of diarrhoea in 20 - 40% of hospitalised children, regardless of country of origin or socio-economic status.10 Our finding that almost 40% of cases of diarrhoea were caused by rotavirus is similar to prevalences of 32%, and 36 -37% in hospitalised patients, in Canada.11,12 An exception is a study that showed a prevalence of 72% at the height of the rotavirus season in Canada.10
The prevalences of diarrhoea (55.5% v. 45.5%) and rotavirus infection (44.3% v. 29.3%) were both higher in boys than in girls. This is a similar gender difference to that found in a Canadian study (55.6% male v. 44.4% female).12 Similar findings are reported from West Africa, where at times the prevalence of rotavirus infection is 20% higher in boys than girls, which corresponds to the 55%:45% differential mentioned above8 (it is possible that boys may be taken for medical attention more frequently than girls). Although these differences did not reach statistical significance in our study, it is intriguing that this gender proportion occurs in many studies.
In most countries diarrhoea occurs year-round, and this is also true of rotavirus infections. However, there is usually a well-known diarrhoea season, and within this a period of increased rotavirus prevalence. For example, in South Africa the rotavirus season has been identified in 14 studies to be in early autumn (March), autumn (March to May) or winter (June to August), while the dry season inland spans the period of mid-autumn to winter.13 In Botswana the rotavirus season was reported to be during June to August, the cool, dry winter season.14 In Ghana there are peaks of rotavirus prevalence in mid-July to September and in January to February,4,15 while the dry season is in July to September and in January. The rotavirus season is also associated with lower daily minimum temperatures.15 In Nigeria rotavirus is present throughout the year, but with a much higher prevalence in the dry season (October to April),4,8 with an average of 36%, than in the rainy season, with an average of 20%.8
In Sierra Leone and neighbouring countries the inter-tropical belt of cloud and rain moves southwards during the rainy season (May to October), when the relative humidity is also high.16 As temperatures hardly vary during the year there are no traditional summers or winters. We found that the highest rotavirus incidence was from July to September, with the peak in August, coinciding with the rainy season (average precipitation in June 302 mm, in July 894 mm, in August 902 mm, in September 610 mm, and in October 310 mm).16 Logistical and financial constraints made it impossible to extend the study to the period from January to June; however, the diarrhoea season falls during the second half of the year, coinciding with the rainy season (personal communication, Dr M A S Jalloh).
The presenting complaints of both the rotavirus-positive and rotavirus-negative groups included diarrhoea. Vomiting, well recognised as a sign of rotavirus infection, was present in all our rotavirus cases, but absent in about a fifth of the rotavirusnegative cases. The rotavirus-positive patients had more severe diarrhoea than those whose diarrhoea was caused by other pathogens, as evidenced by a higher severity score and a larger proportion with moderate dehydration (although the latter difference was not significant). Higher severity scores in a Ghanaian study were also associated with rotavirus infection.9
A third of the sample was underweight for age, which is a common finding in Africa. The prevalence of protein energy malnutrition (kwashiorkor, marasmus and marasmic kwashiorkor) was 8.5%. While this is unacceptably high, Sierra Leone is a country recovering from a devastating civil war so this figure is perhaps not surprising. The fact that protein energy malnutrition was missed in 7 cases indicates that hospital medical staff need training in this aspect of clinical practice. Furthermore, the underweight-for-age group also needs attention.
There was no significant difference between the rotavirus group and the control group with regard to the various systemic signs. The observed signs are nonspecific indicators of several diseases, e.g. generalised lymphadenopathy, fever and pallor could be due to several infections including protozoal, bacterial or viral agents. Cardiovascular and central nervous system signs could be due to any disease condition that is capable of causing moderate to severe dehydration. Despite case reports indicating possible central nervous system involvement of rotavirus infection,17,18 we found very few central nervous system signs in our rotavirus-positive group. The observed changed level of consciousness in 14.7% of rotavirus-positive patients could be due to their dehydration or imbalances in their serum electrolyte levels. Clinical manifestations of respiratory tract infection often precede or coincide with rotavirus gastroenteritis in infants and children. Rotavirus may occasionally be one of the agents causing both upper and lower respiratory infections, and it has been suggested that rotavirus infection may be transmitted via the respiratory route,19 although there has been no conclusive evidence of such transmission.
We thank Dr M A S Jalloh, Superintendent of the Ola During Children's Hospital in Sierra Leone, for his help in enabling the study, Dr J Dewar, formerly of the MRC/Medunsa Diarrhoeal Pathogens Research Unit, for his co-operation and support, the Department of Health of Sierra Leone for granting paid leave to Dr Sawyerr, the African Bank for a grant partially supporting Dr Sawyerr, and Medunsa for financial support.
References
1. World Health Organisation. Rotavirus vaccines. Wkly Epidemiol Rec 2007; 82(32): 285-296. [ Links ]
2. Parashar UD, Bresee JS, Gentsch JR, Glass RI. Synopses: Rotavirus. Emerg Infect Dis 1998; 4: 561-570. [ Links ]
3. Parashar UD, Gibson CJ, Bresee JS, Glass RI. Rotavirus and severe childhood diarrhea. Emerg Infect Dis 2006; 12: 304-306. [ Links ]
4. Cunliffe NA, Kilgore PE, Bresee JS, et al. Epidemiology of rotavirus diarrhoea in Africa: a review to assess the need for rotavirus immunization. Bull World Health Organ 1998; 76(5): 525-537. [ Links ]
5. Molbak K, Fischer TK, Mikkelsen CS. The estimation of mortality due to rotavirus in sub- Saharan Africa. Vaccine 2000; 19: 393-395. [ Links ]
6. Fischer TK, Bresee JS, Glass RI. Rotavirus vaccines and the prevention of hospital-acquired diarrhea in children. Vaccine 2004; 22(1): S49-54. [ Links ]
7. Gomwalk NE, Gosham LT, Umoh UJ. Rotavirus gastroenteritis in paediatric diarrhoea in Jos, Nigeria. J Trop Pediatr 1990; 36: 52-55. [ Links ]
8. Gomwalk NE, Umoh UJ, Gosham LT, Ahmad AA. Influence of climatic factors on rotavirus infection among children with acute gastroenteritis in Zaria, Northern Nigeria. J Trop Pediatr 1993; 39: 293-297. [ Links ]
9. Binka FN, Anto FK, Oduro AR, et al. Incidence and risk factors of paediatric rotavirus diarrhoea in Northern Ghana. Trop Med Int Health 2003; 8(9): 840-846. [ Links ]
10. Rivest P, Proulx M, Lonergan G, Lebel MH, Bédard L. Hospitalisations for gastroenteritis: the role of rotavirus. Vaccine 2004; 22: 2013-2017. [ Links ]
11. Ford-Jones EL, Wang E, Petric M, Corey P, Moineddin R, Fearon M. Hospitalisation for community-acquired rotavirus-associated diarrhea. Arch Pediatr Adolesc Med 2000; 154: 578-585. [ Links ]
12. Waters V, Ford-Jones EL, Petric M, Fearon M, Corey P, Moineddin R. Etiology of community-acquired pediatric viral diarrhea: a prospective longitudinal study in hospitals, emergency departments, pediatric practices and child-care centers during the winter rotavirus outbreak. Pediatr Infect Dis J 2000; 19(9): 843-848. [ Links ]
13. Steele AD, Peenze I, De Beer MC, et al. Anticipating rotavirus vaccines: epidemiology and surveillance of rotavirus in South Africa. Vaccine 2003; 21: 354-360. [ Links ]
14. Basu G, Rossouw J, Sebunya TK, et al. Prevalence of rotavirus, adenovirus and astrovirus infection in young children with gastroenteritis in Gabarone, Botswana. East Afr Med J 2003; 652-655. [ Links ]
15. Armah GE, Mingle JAA, Dodoo AK, et al. Seasonality of rotavirus infection in Ghana. Ann Trop Pediatr 1994; 14: 223-230. [ Links ]
16. BBC weather. Country guide: Sierra Leone. BBC Web Page. http://www.bbc.co.uk/weather/world/country_guides/results.shtml?tt=TT000540 (accessed 10 January 2008). [ Links ]
17. De Villiers FPR, Steele AD, Driessen M. Central nervous system involvement in neonatal infection. Ann Trop Pediatr 2003; 23: 309-312. [ Links ]
18. Lynch M, Lee B, Azimi P, et al. Rotavirus and central nervous system symptoms: Cause or contaminant? Case reports and review. Clin Infect Dis 2001; 33: 932-938. [ Links ]
19. Azevedo MS, Yuan L, Jeong KI, et al. Viraemia and nasal and rectal shedding of rotavirus in gnotobiotic pigs inoculated with human rotavirus. J Virol 2005; 97(9): 5428-5436. [ Links ]
Accepted 6 January 2009.
Corresponding author: F P R de Villiers (johnchild@medunsa.ac.za)














