Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.99 n.2 Pretoria Feb. 2009
ORIGINAL ARTICLES
Oesophageal ulceration in HIV-infected patients
D P EpsteinI; M LocketzII
IMB ChB, DCH (SA), FCP (SA), Cert Gastroenterol (SA). Department of Medicine, Division of Gastroenterology, Groote Schuur Hospital and University of Cape Town
IIMB ChB, FCPath (SA) Anat. Division of Anatomical Pathology, National Health Laboratory Service, and University of Cape Town
ABSTRACT
OBJECTIVE: To determine the aetiology of oesophageal ulceration in HIV-infected patients.
DESIGN: A retrospective clinical, endoscopic and histopathological analysis of patients with confirmed HIV infection and an oesophageal ulcer diagnosed on endoscopy.
SETTING: A tertiary referral, gastrointestinal clinic in Cape Town.
RESULTS: Fifty-one patients with HIV infection and oesophageal ulceration were seen from January 2001 to December 2007. Median CD4 count was 26 cells/µl. Mean age was 35.5 years. Sixty per cent of patients were female. Forty-nine per cent of oesophageal ulcers were idiopathic while 23% were caused by cytomegalovirus infection. The remainder were due to miscellaneous causes.
CONCLUSION: A surprisingly small number of patients with HIVassociated oesophageal ulceration were seen during the study period. This may reflect local referral practices or the fact that patients with severe immunosuppression succumb before developing oesophageal ulcers. As in other series, idiopathic oesophageal ulcers and cytomegalovirus ulcers made up the majority of cases. Correct biopsy technique and appropriate histological and microbiological investigations are associated with improved diagnostic yield in these patients.
Oesophageal ulceration is a well-recognised manifestation of HIV infection. Oesophageal ulcers most often occur with advanced immunosuppression but have also been described in patients undergoing HIV seroconversion.1 Ulceration usually occurs in the mid- to lower third of the oesophagus and presents with odynophagia, dysphagia and spontaneous retrosternal chest pain. Complications such as bleeding, stricture, perforation or fistula formation are well described. In most series cytomegalovirus (CMV) and idiopathic oesophageal ulceration are the most common causes; however, a broad differential of infectious, neoplastic and other diagnoses must be considered in each patient. In some cases more than one cause of ulceration may be present. Oesophageal ulceration is responsible for significant morbidity, impacts on patient nutrition and ability to adhere to oral treatment regimens, and is associated with poor survival.2
No data exist on the aetiology of oesophageal ulceration in South African HIV-infected patients. Oesophageal candidiasis is the most common oesophageal disorder, and accepted practice is to treat patients with oesophageal symptoms with empiric oral azole therapy for 7 - 14 days. It is mandatory that upper gastrointestinal endoscopy be performed in patients who fail to respond to antifungal therapy. In South Africa endoscopy is a limited resource and not readily available to the majority of HIV infected patients.
We aimed to identify the causes of oesophageal ulceration in our patient population.
Study design
From 1 January 2001 to 31 December 2007, all patients with confirmed HIV infection and oesophageal symptoms who were referred to the Groote Schuur Hospital Gastrointestinal Clinic for diagnostic upper endoscopy were considered eligible. Only cases with confirmed endoscopic oesophageal ulceration, with biopsy, were selected. Oesophageal ulcer was defined as a deep mucosal lesion with undermined edges. Sections from each specimen were prepared with haemotoxylin and eosin (H&E), periodic acid Schiff/Alcian blue, Grocott and Ziehl-Neelsen (ZN) stains and with antibody to CMV (Dako, Denmark) and reviewed by ML. TB culture was performed in certain cases. Clinical records were reviewed. The University of Cape Town (UCT) Faculty of Health Sciences Ethics Committee approved the study, which was funded by the Divisions of Gastroenterology and Anatomical Pathology, UCT.
Results
Fifty-one patients were seen: 31 (60%) were female and 20 (40%) were male. Their mean age was 35.5±6.9 years. The median time to endoscopy after development of symptoms was 30 days (range 1 - 365 days). The CD4 count ranged from 1 to 446 cells/µl, with a median of 26 cells/µl and an interquartile range (IQR) of 12 - 48 cells/ul.
Eleven patients (21%) had oral lesions at the time of endoscopy, comprising 5 oral candidiasis, 2 herpes simplex virus (HSV) ulcers, and 4 oral aphthous ulcers. Oral aphthous ulcers were associated with idiopathic oesophageal ulcers in all 4 cases. Twenty-five patients (48%) had idiopathic oesophageal ulceration. Of the remaining ulcers, 12 were caused by CMV infection (23%), 3 by Candida (6%), 4 by TB (8%) and 1 by HSV (2%). CMV and TB were diagnosed in 1 case. In 7 cases no ulcer material was present on histological analysis, reflecting inadequate biopsy of the lesion.
Of the 4 patients with a TB oesophageal ulcer, 3 were diagnosed on direct smear whereas 1 was smear-negative but positive on TB culture.
Barium contrast studies were performed on 10 patients for suspected fistulas, and 1 was identified (Fig. 1).
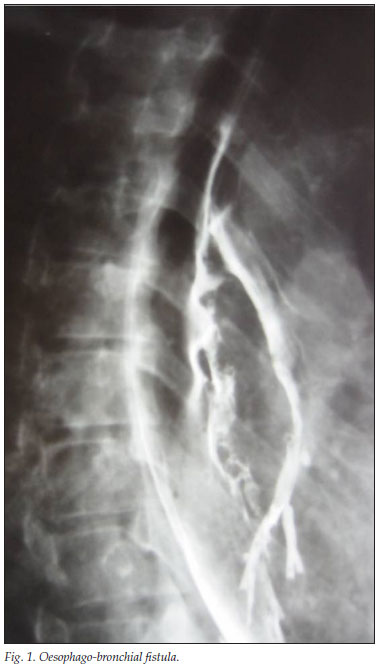
Discussion
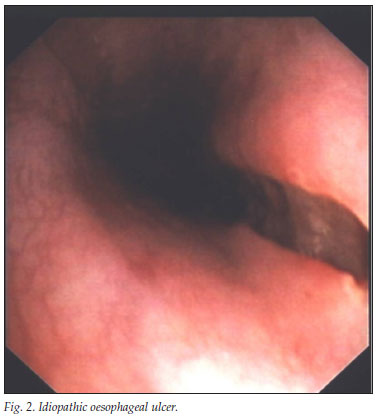
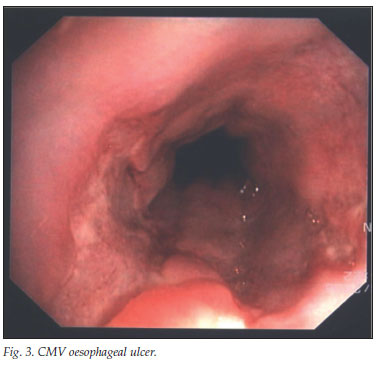
In our series, as in others, the most common oesophageal ulcers were idiopathic (Fig. 2) and those caused by CMV infection (Fig. 3).3,4 In view of the HIV epidemic, it is surprising that so few patients were seen at a tertiary referral gastrointestinal unit, which could be explained by poor referral practices. The latter seems unlikely as we have an open-access endoscopy policy for HIV-infected patients and good community networks. Our study confirms that oesophageal ulceration occurs in patients with severe immunosuppression where 81% of patients had a CD4 count <100 cells/µl. South African HIV-positive patients may succumb to TB and other opportunistic infections before achieving this degree of immunosuppression.
Oropharyngeal lesions were seen in 21% of patients with oesophageal ulceration but this was only predictive of the aetiology of the oesophageal lesion in patients with idiopathic ulcers; however, only 16% of our idiopathic ulcer group had a simultaneous oral ulcer. Oropharyngeal ulceration was found in 11% of 124 HIV-associated oesophageal ulcer patients.5 We also found oral pathology an insensitive indicator of the presence and/or aetiology of oesophageal ulceration.
Tuberculous oesophageal ulceration was identified in 4 (8%) cases. In Brazil, 17% of oesophageal ulcers in HIV-positive patients were due to TB.6 Because of the high incidence of TB in HIV-infected people in South Africa, Ziehl-Neelsen staining and TB culture should be performed on all patients.
HSV-induced ulceration of the oesophagus is common in transplant patients but less common in HIV-infected patients,7 with 1 case in our series. We did not include HSV immunostaining but, since the inclusions are easily recognised morphologically, this omission was probably not responsible for the low number identified.
We had no peptic oesophageal ulceration, neoplastic ulceration or pill-associated ulceration. Advanced HIV disease is associated with hypochlorhydria, and peptic ulceration is unusual in this setting.7
Candida was identified in 3 ulcers (6%), which may represent colonisation of an idiopathic ulcer since the organisms were seen in the ulcer slough rather than invading tissue.
In 7 cases, biopsy material revealed no features of ulceration reflecting inadequate biopsy of the lesion. Wilcox and colleagues employed a biopsy technique to obtain large mucosal samples in their studies of HIV-related oesophageal ulcers.4,8 The 'turn-and-suction' technique,9 initially devised to obtain larger samples in patients undergoing Barrett's oesophagus surveillance, produces larger mucosal samples without complications such as bleeding or perforation.
No standardisation in terms of the number and technique of biopsies was evident in our series. The cytopathic effect of CMV is most notable in infected endothelial cells found within granulation tissue in the ulcer base. By comparison, HSV is more likely to involve squamous epithelial cells present in biopsies from the ulcer edge.4 Correct biopsy technique and sample number is important to establish an accurate diagnosis in patients with HIV-related oesophageal ulceration.
Oesophageal ulceration occurs in patients with severe immunosuppression, and antiretroviral therapy is the cornerstone of therapy. Inducing ulcer healing, preventing ulcer recurrence, analgesia and maintaining nutrition are other components of therapy.
Treatment options for idiopathic oesophageal ulceration include oral, intravenous and intralesional steroids. Systemic steroids are effective in symptomatic and endoscopic healing of idiopathic ulcers;7,10 however, relapse on withdrawal of therapy and the increased risk of opportunistic infections are limiting factors. Oral thalidomide is effective in healing idiopathic oesophageal ulcers10,11 but is associated with significant sideeffects.12
We commence antiretroviral therapy in all patients with oesophageal ulceration who are not already on treatment. Identified specific opportunistic infections are treated. Nutrition is maintained with an oral puree diet or a liquid nutritional supplement. Fine-bore nasogastric tube feeding is used for patients unable to maintain nutrition orally. None in our series required percutaneous endoscopic gastrostomy tube feeding. Oral Mist Morphine is routinely used for analgesia.
Oesophageal ulceration is a debilitating manifestation of advanced HIV infection and is most often due to idiopathic ulceration or CMV. Endoscopy with adequate biopsy of the lesion is the investigation of choice. Appropriate histological and microbiological tests, including TB culture, should be performed in all cases. Contrast studies should be performed if complications such as a bronchial fistula or stricture are suspected. Pharmacological intervention should include antiretroviral therapy and treatment of the causative opportunistic infection, if identified. In addition pain management and maintenance of nutrition are important in achieving a successful outcome.
References
1. Siegmund B, Moos V, Loddenkemper UW, et al. Esophageal giant ulcer in primary human immunodeficiency virus infection is associated with an infiltration of activated T cells. Scand J Gastroenterol 2007; 42: 890-895. [ Links ]
2. Connolly GM, Hawkins D, Harcourt-Webster JN, et al. Oesophageal symptoms, their causes, treatment, and prognosis in patients with the acquired immunodeficiency syndrome. Gut 1989; 30: 1033-1039. [ Links ]
3. Bonacini M, Young T, Laine L. The causes of esophageal symptoms in human immunodeficiency virus infection. Arch Intern Med 1991; 151: 1567-1572. [ Links ]
4. Wilcox CM, Straub RF, Schwartz DA. Prospective evaluation of biopsy number for the diagnosis of viral esophagitis in patients with HIV infection and esophageal ulcer. Gastrointest Endosc 1996; 44: 587-593. [ Links ]
5. Wilcox CM, Straub RF, Clark WS. Prospective evaluation of oropharyngeal findings in human immunodeficiency virus-infected patients with esophageal ulceration. Am J Gastroenterol 1995; 90: 1938-1941. [ Links ]
6. Calore EE, Cavaliere JM, Perez NM, et al. Esophageal ulcers in AIDS. Pathologica 1997; 89(2): 155-158. [ Links ]
7. Wilcox CM. Esophageal disease in the acquired immunodeficiency syndrome: etiology, diagnosis, and management. Am J Med 1992; 92: 412-421. [ Links ]
8. Wilcox CM, Schwartz DA, Clark WS. Esophageal ulceration in human immunodeficiency virus infection. Ann Intern Med 1995; 122: 143-149. [ Links ]
9. Levine DS, Reid BJ. Endoscopic biopsy technique for acquiring larger mucosal samples. Gastrointest Endosc 1991; 37: 332-337. [ Links ]
10. Kotler DP, Reka S, Orenstein JM, et al. Chronic idiopathic ulceration in the acquired immunodeficiency syndrome. J Clin Gastroenterol 1992; 15(4): 284-290. [ Links ]
11. Laine L, Bonacini M. Esophageal disease in human immunodeficiency virus infection. Arch Intern Med 1994; 154: 1577-1582. [ Links ]
12. Jacobson JM, Spritzler J, Fox L, et al. Thalidomide for the treatment of esophageal aphthous ulcers in patients with human immunodeficiency virus infection. J Infect Dis 1999; 180: 61-67. [ Links ]
13. Jacobson JM, Greenspan JS, Spritzler J, et al. Thalidomide in low intermittent doses does not prevent recurrence of human immunodeficiency virus-associated aphthous ulcers. J Infect Dis 2001; 183: 343-346. [ Links ]
Accepted 30 June 2008.
Corresponding author: D Epstein (david@gastro-enterology.co.za)














