Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.98 n.12 Pretoria Dec. 2008
ORIGINAL ARTICLES
Factors predicting walking intolerance in patients with peripheral arterial disease and intermittent claudication
B ParrI; T D NoakesII; E W DermanIII
IMSc (Med). Department of Sport Management, Cape Peninsula University of Technology, Cape Town
IIMB ChB, MD, DSc, FACSM. Faculty of Health Sciences, University of Cape Town
IIIMB ChB, PhD, FACSM. Faculty of Health Sciences, University of Cape Town
ABSTRACT
OBJECTIVE: To determine which physiological variables conduce to walking intolerance in patients with peripheral arterial disease (PAD).
DESIGN: The physiological response to a graded treadmill exercise test (GTT) in patients with PAD was characterised.
SETTING: Patients were recruited from the Department of Vascular Surgery, Groote Schuur Hospital, Cape Town.
SUBJECTS: Thirty-one patients diagnosed with PAD were included in the study.
OUTCOME MEASURES: During a GTT, peak oxygen consumption (VO2peak), peak minute ventilation (VEpeak), peak heart rate and peak venous lactate concentrations were measured and compared with those from a comparison group. Ankle-brachial index (ABI) was measured at rest and after exercise. During the GTT, maximum walking distance (MWD) and pain-free walking distance (PFWD) were measured to determine walking tolerance.
RESULTS: Peak venous lactate concentrations did not correlate significantly with either PFWD (r=-0.08; p=0.3) or MWD (r=-0.03; p=0.4). Resting ABI did not correlate with either MWD (r=0.09; p=0.64) or PFWD (r=-0.19; p=0.29). Subjects terminated exercise at significantly (p<0.05) lower levels of cardiorespiratory effort and venous lactate concentrations than did a sedentary but otherwise healthy comparison group: peak heart rate 156±11 v. 114±22 beats per minute (BPM); p=0.001; and peak venous lactate concentration 9.7±2.7 mmol/l v. 3.28±1.39 mmol/l; p=0.001.
CONCLUSION: Perceived discomfort in these patients is not caused by elevated blood lactate concentrations, a low ABI or limiting cardiorespiratory effort but by other factors not measured in this study.
Peripheral arterial disease (PAD) is a complex disease with patients typically suffering from hypertension, diabetes and coronary artery disease (CAD). Risk factors for PAD include smoking, hypercholestrolaemia, male gender and age.1 Patients with PAD are walking intolerant.
The cause of walking intolerance in patients with PAD is not clear. It has been suggested that a compromised oxidative metabolism accelerates lactate accumulation in the exercising skeletal muscles, inducing the pain of intermittent claudication.2,3 Studies have shown that improvements in walking tolerance with exercise training are associated with increases in the concentration of skeletal muscle oxidative enzymes2,4,5 and improved oxygen extraction by the trained skeletal muscles.6,7 These findings have served to reinforce the hypothesis that skeletal muscle anaerobiosis causing increased lactate production in the ischaemic exercising skeletal muscles causes the pain of claudication.
Other researchers have suggested that walking intolerance results primarily from decreased blood flow to the lower limb so that improvements in blood flow as a result of a training-induced increase in collateral circulation enhance walking tolerance in patients with PAD.8-10 Therefore the ankle brachial index (ABI) is a commonly used clinical measure to predict the functional capacity of patients with this complaint.
Our main objective was to examine the relationship between peak physiological parameters measured during symptom-limited maximal exercise and the walking tolerance of patients with PAD. We postulated that certain physiological parameters (in particular, high venous blood lactate concentrations and a low ABI, both indicating a reduced capacity for blood flow to the exercising skeletal muscles, thus causing an early onset of ischaemia) would predict their walking distance. A secondary objective was to report the demographical and medical characteristics of 31 patients with PAD.
Methods
Participants
Thirty-one patients with PAD were recruited from the Department of Vascular Surgery at Groote Schuur Hospital, and from Kingsbury Hospital, Claremont, Cape Town. All patients had PAD with intermittent claudication, as diagnosed by a vascular surgeon using duplex flow Doppler and/or segmental Doppler pressures. For inclusion, patients had to develop intermittent claudication during a screening treadmill test and to have an ABI at rest of less than 0.97 or a fall of the ABI to <0.85 during exercise. Patients were excluded if any of the following were present: absence of PAD; rest pain or tissue loss; limitations of exercise tolerance caused by conditions other than claudication (e.g. severe CAD, dyspnoea, severe arthritis); and active cancer, liver or renal disease. Patients gave their written informed consent. The study protocol was approved by the Ethics and Research Committee of the Faculty of Health Sciences of the University of Cape Town.
Measurements
On the first visit to the laboratory, a full anthropometric analysis was performed for determination of body composition, a full medical history was taken and medical records were obtained from the vascular surgeon and cardiologist. Patients were familiarised with the treadmill protocol.
Exercise test
On the second visit, patients performed a graded treadmill exercise test (GTT) to measure physiological variables during maximal exercise. During the GTT, the speed was held constant at 3.2 km/h with an initial 2% gradient.11 The gradient was then increased by 2% every 2 minutes. If unable to walk at that speed, subjects were tested using the Modified Bruce protocol in which the treadmill speed was held constant at 1.7 km/h. Heart rate and brachial artery blood pressure were monitored every 2 minutes. Patients reported their level of perceived pain according to a perception of pain scale12 that uses simple-to-understand verbal expressions which accurately describe sensations of perceived peripheral pain. Pain-free walking distance (PFWD) was recorded as the distance walked before the patient first reported calf pain during exercise. Maximum walking distance (MWD) was defined as the distance covered on the treadmill until the patient voluntarily terminated exercise as a result of severe claudication pain that could not be tolerated.
During exercise, inspiratory ventilation rate (VE), oxygen uptake (VO2) and respiratory exchange ratio (RER) were measured over 15-second intervals by breath-by-breath Oxycon Alpha Analyzer (Oxycon Alpha, Enrich Jaeger, Wuerzburg, Germany). Before each test, the gas meter was calibrated with a Hans Rudolph 3-litre syringe (Vacumed, Ventura, California), and the analysers were calibrated with room air and a 4% CO2 -16% O2 - 80% N2 gas mixture. The reliability of the Oxycon Alpha Analyzer was tested weekly by burning ethanol (99% analytical reagent, Associated Chemical Enterprises, Glenvista, South Africa) as a reference. Heart rate during the GTT was recorded using an electrocardiogram monitor (Cardio Perfect 3.3, Rijswijk, the Netherlands) with self-adhering electrodes placed in a modified 10-electrode (Mason-Likar) configuration.8
Blood pressure
Before the treadmill test, subjects remained supine in a quiet environment for 15 minutes and the resting brachial blood pressure (Korotkoff phase I and IV) was measured and recorded by means of audible sphygmomanometry using a calibrated mercury column sphygmomanometer with an appropriately sized cuff. Simultaneously, another tester measured left and right ankle pressures consecutively with a Doppler (Huntleigh Technology PLC 1997 Dopplex Advanced Pocket Dopplers, UK) and blood pressure cuffs affixed just above the ankles. The Doppler probe was placed over the posterior tibial or dorsalis pedis artery. The cuff was inflated until the Doppler sound disappeared, and slowly deflated until the sound returned. The ankle systolic pressure was measured when the sound returned. ABI was calculated for each patient by dividing the ankle pressure reading by the brachial pressure reading. The procedure was repeated immediately after exercise.
Blood sample analysis
Before the start of the treadmill test, a resting venous blood sample was drawn from each patient; 2 ml of blood was drawn directly into a sodium oxalate-lined glass test tube and immediately placed on ice for later analysis of plasma lactate concentrations; and a 2 ml blood sample was collected 3 minutes after exercise when the blood lactate concentration peaks.13
The 2 ml blood samples in the sodium oxalate test tubes were centrifuged using a Sigam 302-K centrifuge (Munich, Germany) at 3 000 rpm for 10 minutes at 4°C. Blood lactate concentrations were measured in plasma by means of an enzymatic kit technique (bioMerieux SA, lactate PAP 69280, Marcy l'Etoille, France).
Statistical analysis
Data are presented as means ±SD. Correlation coefficients within the patient population were calculated by means of Pearson's product moment correlation coefficient for continuous data using Statistica (StatSoft Inc., Tulsa, USA). Levels of significance were calculated at the 0.05 confidence levels. In the cases of correlations, correlation coefficients were denoted as r and the sample size as N. A dependent t-test was used to assess differences between pre-exercise venous lactate concentrations and post-exercise lactate concentrations. Ratings of pain taken every 2 minutes during the exercise tests were converted to a single variable for each patient by plotting an area under the curve for each patient. The data were normalised by expressing the pain measure (Y axis) relative to 100% of elapsed time (X axis). Independent t-tests were used to detect differences between means of this sample group and the comparison group from this laboratory.
Results
General and medical characteristics of patients are detailed in Tables I and II.

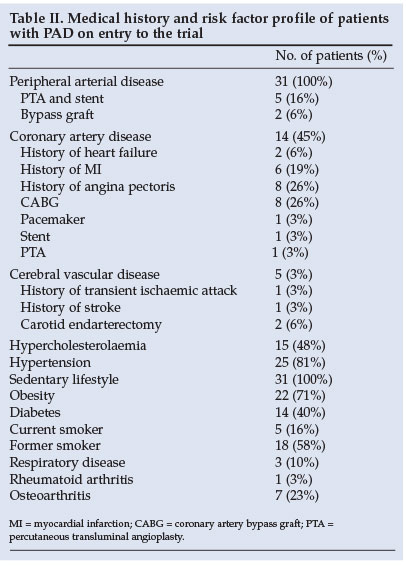
The mean MWD during the GTT was 317 m; pain-free walking distance was 98 m (Table I). Of significance is that 45% of patients with PVD also had clinical evidence for CAD, 81% had hypertension, 48% had hypercholesterolaemia and 40% had diabetes (Table II).
We were unable to develop an age-matched control group of apparently healthy individuals without PVD. The findings were therefore compared with data collected from a previous study in this laboratory of a sedentary but otherwise healthy middle-aged control group (CONT23 - Table III). The CONT group had higher peak VO2, VE, HR and RER values and peak lactate concentrations than patients with PVD (Table III).

There was no correlation between peak post-exercise venous lactate concentrations and PFWD (r=-0.08; p=0.33) or MWD (r=-0.03; p=0.44) during the GTT (Figs 1a and 1b).
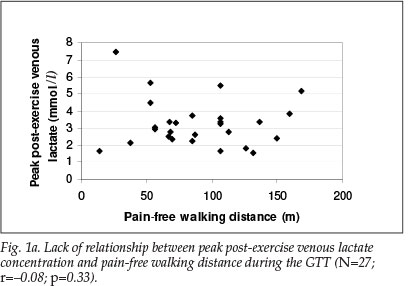

There was a trend for ABI to drop after the GTT (0.6±0.4 v. 0.4±0.4 pre-exercise to post exercise, p=0.056) (Table I). However, resting ABI did not correlate with MWD (r=0.08; p=0.3) or pain-free walking distance (r=-0.19; p=0.15) (Figs 2a and 2b); nor was there any relationship between post-exercise ABI and walking tolerance during the GTT. Predictably, VO2peak (r=0.68; p=0.001) and Vepeak (r=0.5; p=0.005) correlated significantly with MWD during the treadmill exercise test.

No significant correlations were found between age, BMI, fat percentage, duration of disease, pack-years of cigarette smoking and total pain, and walking tolerance during the GTT; i.e. there was no relationship between these variables and walking tolerance. There was, however, a weak but significant correlation between mass and PFWD during the GTT (r=0.39; p=0.03).
A correlation analysis was performed between total pain perceived on the GTT and peak venous blood lactate concentration and total pain perceived on the GTT and ABI. There was no correlation between total pain perceived on the GTT and peak venous blood lactate or with ABI.
Discussion
We found that resting ABI did not predict PFWD or MWD or perception of pain during the GTT. Therefore, functional capacity was independent of ABI, suggesting that ABI should not be used to predict exercise tolerance or functional improvement in patients with PAD. This finding agrees with a report that improvements in walking performance in patients with PAD were not accompanied by improvements in ABI.14 If ABI cannot predict functional capacity, the use of a GTT to monitor functional capacity in patients with PAD may be more valuable than reliance on the ABI alone.
We also found that peak post-exercise venous lactate concentrations did not correlate with PFWD or MWD (Figs 1a and 1b). Therefore, patients with the highest post-exercise venous lactate concentrations did not have the shortest PFWD or MWD, as might be expected if venous lactate concentrations are related to the development of leg pain in this condition. Moreover, peak venous lactate concentrations of patients with PAD in this study were lower (Table III) than in a control group. There was no correlation between total pain perceived on the GTT and peak venous blood lactate.
This finding is of interest as exercise physiologists and clinicians studying PVD commonly believe that anaerobic glycolysis and the resultant increase in lactate concentrations in the ischaemic muscles and blood explains why these patients develop the pain of intermittent claudication and why their exercise tolerance is so impaired.2,3,5 For example, Tan et al. stated: 'In patients with peripheral vascular disease, increasing the workload causes an inequality in the supply of and demand for oxygen. Aerobic generation of adenosine triphosphate (ATP) becomes inadequate and anaerobic metabolism predominates. The result (of exercise) is an increase in lactic acid production, and a depletion of ATP and creatine phosphate (CP), leading to pain.'3
This explanation stems from the popular theory suggesting that fatigue develops as a result of anaerobiosis in the exercising skeletal muscles and accelerates glycolysis.15 The by-product of anaerobic glycolysis is lactate accumulation in the skeletal muscles and blood. Therefore, many studies of patients with PAD investigated peripheral adaptations that may delay the onset of anaerobic glycolysis and therefore delay the onset of claudication and increase walking tolerance. These adaptations include increases in the concentration of skeletal muscle oxidative enzymes2,4,5 and improved oxygen extraction by the skeletal muscles.6,8 Lundgren et al. reported that '... the results ... support the contention that an improved oxidative capacity is beneficial in improving PFWD. But as long as there is blood flow restriction, there will be a point when flow becomes the limiting factor producing hypoxia, lactate accumulation and pain.'2
It can be argued that low blood lactate concentrations could be associated with elevated muscle lactate concentrations in patients with PAD. If this is true, lactate accumulating in the skeletal muscle, and not in the blood, would be the direct cause of pain and should be measured rather than blood lactate concentrations to evaluate the role of lactate in the production of pain in this condition. However, Pernow et al. reported that peak muscle lactate concentrations in controls and in patients with PAD were equally high (13.7±1.6 v. 12.1±2.3 mmol/ kg).16 While these data seem to differ from our own, since we would have expected muscle lactate concentrations to be low (in keeping with the low blood lactate concentrations in our patients), Pernow et al.'s data do not support the theory that lactate 'leads to pain' (Tan et al.) as their control group did not experience claudication during maximal exercise despite similar peak muscle lactate concentrations to patients with PAD.3,16
In addition, our patients with PAD terminated exercise at lower heart rates, lower RER values and lower venous lactate concentrations than a sedentary control group. Patients with PAD also had lower VO2 and VE values than a sedentary control group. Therefore, perceived discomfort in these patients was not caused by elevated blood lactate concentrations or by limiting cardiorespiratory effort, but must be caused by other factors not measured.
Lastly, patients with PAD in this study exhibited a variety of other co-morbid conditions, all of which can be positively influenced by participation in regular physical activity since this reduces all-cause mortality,17 reduces the risk of cardiovascular disease,18,19 lowers resting blood pressure20 and favourably influences the blood lipid profile.21,22
Conclusion
Patients with PAD terminated exercise at low venous blood lactate concentrations and low levels of cardiorespiratory demand compared with a control group. Furthermore, no measured variable could predict walking distance in the PAD patients. Therefore, perceived discomfort and factors determining the walking distance of these subjects could not be explained on the basis of the traditional measurements used to predict exercise performance in healthy subjects or in patients with PAD. Future research should consider alternative explanations for this unexpected finding.
References
1. Murabito JM, Agostino RB, Silbershaatz H, et al. Intermittent claudication: A risk profile from the Framingham Heart Study. Circulation 1997; 96: 44-49. [ Links ]
2. Lundgren F, Dahloff AG, Chersten T, et al. Muscle enzyme adaptation in patients with peripheral arterial insufficiency: spontaneous adaptation, effect of different treatments and consequences on walking performance. Clin Sci 1989; 77: 485-493. [ Links ]
3. Tan KH, De Cossart AL, Edwards PR. Exercise training and peripheral vascular disease. Br J Surg 2000; 87: 553-562. [ Links ]
4. Holm J, Dahloff AG, Bjorntorp P, et al. Enzyme studies in muscles of patients with intermittent claudication. J Clin Lab Invest 1973; 31: 201-205. [ Links ]
5. Hiatt WR, Regensteiner JG, Wolfel EE, et al. Effect of exercise training on skeletal muscle histology and metabolism in peripheral arterial disease. J Appl Physiol 1996; 81: 780-788. [ Links ]
6. Zetterquist S. The effect of active training on the nutritive blood flow in exercising ischemic legs. Scan J Clin Lab Invest 1970; 25: 101-111. [ Links ]
7. Sorlie D, Myhre K. Effects of physical training in intermittent claudication. Scand J Clin Lab Invest 1978; 38: 217-222. [ Links ]
8. American College of Sports Medicine. Guidelines for Exercise Testing and Prescription. 5th ed. London: Williams & Wilkins, 1995. [ Links ]
9. Jonason T, Ringqvist I. Effect of training on the post-exercise ankle blood pressure reaction in patients with intermittent claudication. Clin Physiol 1986; 7: 63-69. [ Links ]
10. Skinner JS, Strandness DE. Exercise and intermittent claudication. Circulation 1967; 36: 23-29. [ Links ]
11. Perakyla T, Tikkanen H, Von Knorring J, et al. Poor reproducibility of exercise test in assessment of claudication. Clin Physiol 1998; 18: 187-193. [ Links ]
12. Lambert MI, Marcus P, Burgess T, et al. Electro-membrane microcurrent therapy reduces signs and symptoms of muscle damage. Med Sci Sports Exerc 2002; 34: 602-607. [ Links ]
13. Kowalchuk JM, Hugenhauser GJ, Lindinger MI, et al. Factors influencing hydrogen ion concentration in muscle after intense exercise. J Appl Physiol 1988; 65(5): 2080-2089. [ Links ]
14. Creasy TA, McMillan PJ, Fletcher EW, et al. Is percutaneous transluminal angioplasty better than exercise for claudication? - Preliminary results from a prospective randomized trial. Eur J Vasc Surg 1990; 4: 135-139. [ Links ]
15. Noakes TD. Physiological models to understand exercise fatigue and the adaptations that predict or enhance athletic performance. Scand J Med Sci Sports 1999; 9: 1-23. [ Links ]
16. Pernow B, Saltin B, Wahren J, et al. Leg blood flow and muscle metabolism in occlusive arterial disease of the leg before and after reconstructive surgery. Clin Sci Mol Med 1975; 49: 265-275. [ Links ]
17. Paffenberger RS, Hyde RT, Wing AL, et al. Physical activity, all-cause mortality, and longevity of college alumni. N Engl J Med 1986; 252: 491-495. [ Links ]
18. Powell KE, Thompson PD, Casperson CK, et al. Physical activity and the incidence of coronary heart disease. Annu Rev Public Health 1987; 8: 253-287. [ Links ]
19. Paffenberger RS, Hyde RT, Wing AL, et al. A natural history of athleticism and cardiovascular health. JAMA 1984; 252: 491-495. [ Links ]
20. Kelley G, McClellan P. Antihypertensive effects of aerobic exercise. A brief meta-analytic review of randomized controlled trials. J Am Coll Cardiol 1989; 13: 241A. [ Links ]
21. Pasquali SK, Alexander KP, Peterson DE. Cardiac rehabilitation in the elderly. Am Heart J 2001; 143: 748-755. [ Links ]
22. Lavie CJ, Milani RV. Effects of cardiac rehabilitation programmes on exercise capacity, coronary risk factors, behavioural characteristics and quality of life in a large elderly cohort. Am J Cardiol 1995; 76: 177-179. [ Links ]
23. Coleman KL. Exercise tolerance and skeletal muscle structure and function in patients with chronic obstructive pulmonary disease. M Sci thesis, University of Cape Town, 1998. [ Links ]
 Correspondence:
Correspondence:
B Parr
(parrb@cput.ac.za)
Accepted 18 July 2008.














