Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.98 no.8 Pretoria Ago. 2008
ORIGINAL ARTICLES
Antioxidant and oxidative stress status in type 2 diabetes and diabetic foot ulcer
Elizabeth Bosede BolajokoI; Kensese Sontin MossandaII; Mpho MoropaneIII; Francis AdeniyiIV; Olubayo AkinosunV; Adesoji FasanmadeVI
IMSc; Department of Chemical Pathology, University of Limpopo, Medunsa Campus, GaRankuwa
IIPhD; Department of Chemical Pathology, University of Limpopo, Medunsa Campus, GaRankuwa
IIIBTech; Department of Anatomical Pathology University of Limpopo, Medunsa Campus
IVPhD; Department of Chemical Pathology, University of Ibadan, Ibadan, Nigeria
VMB BS; Department of Chemical Pathology, University of Ibadan, Ibadan, Nigeria
VIMB BS; Department of Medicine, University of Ibadan
ABSTRACT
OBJECTIVE: Oxidative stress (OS) has been implicated in the aetiology and progression of diabetic complications including diabetic foot ulcer. In this study, the levels of lipid peroxides (LPO) and 8-hydroxy-2'-deoxyguanosine (8-OHdG) as well as the enzymatic antioxidant activities of superoxide dismutase (SOD) and glutathione peroxidase (GPx) in type 2 diabetes mellitus and diabetic foot ulcer subjects were assessed and compared with apparently healthy normal subjects to understand the involvement of OS in the subjects.
METHOD: The abovementioned OS markers were measured in 50 subjects for each of the following groups: type 2 diabetes mellitus (DM), diabetic foot ulcer (DF) and non-diabetic control (NC).
RESULTS: Significant elevated values of LPO (39.86%) and 8-OHdG (45.53%) were found in DM subjects compared with the NC subjects. This increase in both parameters was greater for DF subjects: 80.23% and 53.91% respectively. SOD activities were significantly reduced in DM (14.82%) and DF (4.09%) subjects in contrast with elevated activities of GPx observed in DM (21.87%) and DF (20.94%) subjects. Glycated haemoglobin/fasting plasma glucose (HbA1c/FPG) correlated positively with LPO, 8-OHdG and GPx, whereas a negative correlation was observed for SOD.
CONCLUSION: Increased oxidation subsequent to diabetic conditions induces an over-expression of GPx activity suggesting a compensatory mechanism by the body to prevent further tissue damage in the subjects.
A body of evidence exists concerning the involvement of oxidative stress (OS) in the aetiology of diabetes and its later complications, of which diabetic foot ulcer is one.1 OS arises in cells and tissues through the increased production of reactive oxygen species (ROS) and/or from decreases in the antioxidant defence system.2 Several mechanisms seem to be involved in the generation of OS in the presence of elevated glucose concentrations; they include glucose auto-oxidation, enhanced glucose flux through the polyol pathway, and non-enzymatic and progressive glycation of proteins with consequent increased formation of glucose-derived advanced glycosylation end products (AGEs).3
Under normal physiological conditions, a widespread antioxidant defence system protects the body against the adverse effects of ROS generation. The defence mechanism's efficiency is altered in diabetes and the ineffective scavenging of free radicals may therefore play a crucial role in determining tissue damage in these subjects.4
The present investigation was carried out to assess the levels of lipid peroxides (LPO) (a marker of lipid peroxidation) and 8-hydroxy-2'-deoxyguanosine (8-OHdG) (a marker of DNA damage), as well as the enzymatic antioxidant activities of superoxide dismutase (SOD) and glutathione peroxidase (GPx) in normal control (NC) type 2 diabetes mellitus (DM) and diabetic foot ulcer (DF) subjects, to understand the involvement of OS in diabetic foot ulcers.
Materials and methods
Patients
The study population comprised 50 type 2 DM subjects and 50 DF subjects with Wagner's grade II ulcer classification (i.e. ulcer without abscess or osteomyelitis). Males and non-pregnant/non-lactating females between the ages of 40 and 60 years with HbAlc >6.5% were recruited from the medical ward of University College Hospital, Ibadan, Nigeria, as 'test' groups. In addition, 50 age-matched healthy non-diabetic subjects of both genders with HbAlc <6.5% were selected as a normal control group from among the staff of the same university. Informed consent was sought and obtained from each subject before recruitment into this study, which was approved by the Ethical Committee of the University of Ibadan and the University College Hospital Institutional Review Committee (UI/UCH IRC) (approval number UI/ IRC/03/0096).
Blood sampling
Ten-millilitre aliquots of venous blood drawn after a 10-hour overnight fast were collected in heparinised EDTA or fluoride sample tubes and centrifuged at 3 000 rpm for 10 minutes. Plasma and haemolysate were stored at -80°C until the day of analysis. Antioxidant enzymes (SOD, GPx) were measured in heparinised whole blood, whereas oxidative status parameters (LPO, 8-OHdG) were assessed in EDTA plasma.
Fasting plasma glucose (FPG) and glycated haemoglobin A1c (HbAlc) were measured in plasma from sodium fluoride and EDTA samples respectively, on the day of collection using standard laboratory techniques.
Analyses
Chemical reagents of the highest quality were purchased from Sigma-Aldrich, Germany. LPO concentrations were measured spectrophotometrically at 560 nm using the ferrous oxidation with xylenol orange (FOX VERSION II) assay according to the method of Nourooz-Zadeh et al.5 This method is based on the principle of rapid peroxide-mediated oxidation of Fe2+ to Fe3+ under acidic conditions.
Plasma levels of 8-OHdG were measured at 450 nm on a microplate plate reader using a commercial kit from the Japan Institute for the Control of Aging (Fukuroi, Japan). The method is based on a competitive in vitro enzyme-linked immunosorbent assay for quantitative measurement of this DNA metabolite in tissue, serum and plasma.6
Erythrocyte SOD activity was determined by the method of Arthur and Boyne,7 using a commercial kit obtained from Randox Laboratories, UK. This method uses xanthine and xanthine oxidase (XOD) to generate superoxide radicals which react with 2-(4-iodophenyl)-3-(4-nitrophenol)-5-phenyltetrazolium chloride (INT) to form a red formazan dye. SOD activity is then measured by the degree of inhibition of this reaction spectrophotometrically at 505 nm.
The determination of erythrocyte GPx activity was based on modification of the method of Paglia and Valentine,8 using a commercial kit obtained from Randox Laboratories, UK. This method involves the oxidation of glutathione (GSH) by cumene hydroperoxide catalysed by GPx. In the presence of glutathione reductase (GR) and NADPH, the oxidised glutathione (GSSG) is immediately converted to the reduced form with a concomitant oxidation of NADPH to NADP+. The decrease in absorbance is then measured spectrophotometrically at 340 nm.
Statistical analysis
All data were presented as mean ± standard deviation (SD) for 50 subjects in each group. The Statistical Package for Social Sciences (SPSS) (version 13) was used for statistical evaluation. Significance of differences was determined using one-way analysis of variance (ANOVA). Pearson's correlation coefficient was determined within groups. Statistical significance was set at p-values <0.05.
Results
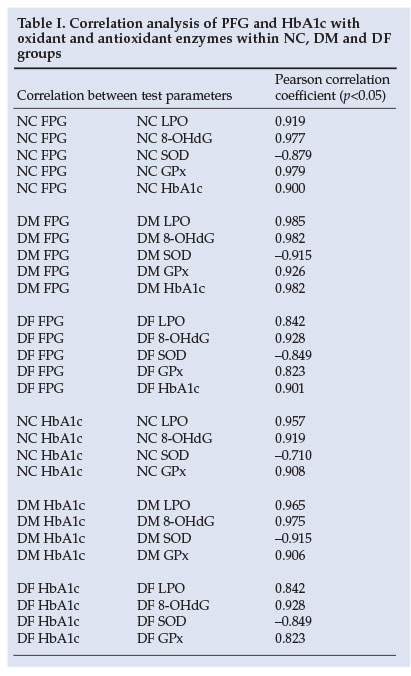
Pearson's correlation analysis of all the groups revealed a strong correlation of FPG and HbAlc with LPO, 8-OHdG and GPx, in contrast to a negative correlation with SOD. As expected, NC-HbAlc correlated positively with NC-FPG (r=0.899). This correlation became stronger in the DM and DF groups, with a correlation coefficient of 0.982 and 0.901 respectively, as shown in Table I (p<0.05). The negative correlation between FPG and SOD (r=-0.879) in the NC group is shown in Fig. 1.
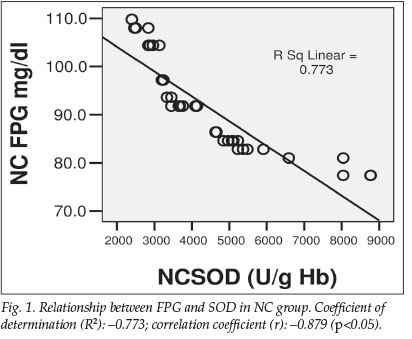
The mean values of FPG and HbA1c were respectively 214.84±65.26 mg/dl and 7.97±2.55% for the DM group. For the DF group, they were 226.93±127.10 mg/dl and 8.40±1.91% respectively, compared with 91.69±9.56 mg/dl and 4.08±0.75% for the NC group, as shown in Fig. 2a.
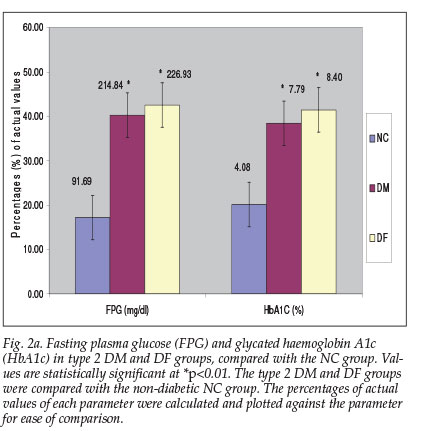
A high level of LPO was observed in the plasma of the DF group (56.61±17.34 µM), compared with the DM group (43.93±16.46 uM) and NC group (31.41±15.95 uM). The extent of oxidation was reflected in the concentration of DNA adduct, 8-OHdG: 49.05±13.79 uM and 46.38±18.03 µM, corresponding to an increase of 53.91% and 45.53% respectively for the DF and DM groups, compared with the NC group (31.87±11.58 uM) (Fig. 2b).
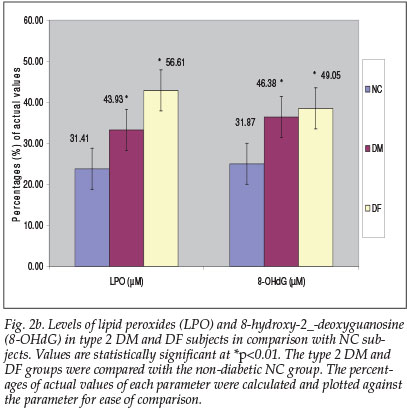
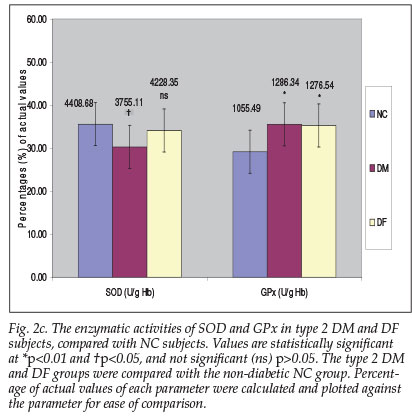
A slight increase in GPx activity was observed in the DM and DF groups (21.87% and 20.94% respectively) in contrast with a substantial decrease in SOD activity in the DF (4.09%) and especially in the DM group with a decrease of 14.82% (p<0.05) (Fig. 2c).
Discussion
During diabetes, persistent hyperglycaemia leads to an increased production of ROS through the glucose autoxidation, sorbitol pathway and non-enzymatic protein glycation. Oxidative stress arises when the production of ROS (which include both oxygen radicals such as superoxide (O2•-), alkoxyl (RO), peroxyl (ROO) and hydroxyl (HO) radicals, and non-radical derivatives of oxygen, namely hydrogen peroxide) exceeds the capacity of the available antioxidant defence system. The excess ROS tends to react with virtually all cell components, resulting in lipid membrane peroxidation, protein denaturation and DNA damage.9
In this study, a high level of HbA1c and FPG was found in both type 2 DM and DF groups, with the latter group having a greater increase as a consequence of poorly controlled glycaemia in these subjects. The correlation coefficient results obtained in this study revealed a strong association between these two parameters (FPG and HbA1c) and the level of oxidants and the activity of antioxidant enzymes in diabetic subjects with and without foot ulcers as well as non-diabetic subjects.
One of the characteristic features of chronic diabetes is lipid peroxidation resulting from excessive reactions of free radicals with polyunsaturated fatty acids (PUFAs) in cell membranes. This lipid peroxidation in turn leads to elevated production of free radicals.10 Lipid peroxide-mediated damage has been observed in both types of DM.
We have indeed confirmed in our study the findings of Santini et al.11 on the increase of plasma LPO levels in diabetic subjects, compared with those of control subjects. In addition to these observations, a substantially elevated level of this parameter was found in DF subjects, which may be due to their increased production of ROS.
As with other biomolecules, deoxyribonucleic acid (DNA) is also susceptible to damage induced by free radicals. An elevated level of 8-OHdG (a marker used for assessing the extent of DNA oxidative damage by free radicals3) was indeed observed in type 2 DM and DF subjects (45.53% and 53.91% respectively), compared with the NC group. This finding is in agreement with the study by Dandona et al.,12 who reported greater oxidative damage to DNA with more increased generation of ROS in both type 1 and type 2 DM patients than normal controls.
Specific enzymatic antioxidant defence systems have evolved to deal with individual ROS. SOD scavenges superoxide radicals by converting them to hydrogen peroxide and molecular oxygen, while GPx converts hydrogen peroxide to oxygen and water.13
Controversial reports on changes in erythrocyte activity of SOD in both type 1 and type 2 diabetes have been published. In some, a decrease9 in the activity was observed, whereas in others an increase14 or no change15 was reported. In this study, however, a substantial decrease in SOD activity (14.82%; p<0.05) in DM and a slight reduction (4.09%; p>0.05) in DF groups were observed, compared with the NC group. The reduction in SOD activity observed in this study is comparable to the work of Bhatia et al.,9 who reported a significant reduction in SOD activity in DM subjects. The observed decrease in SOD activity could have resulted from glycation of the enzyme, which has been reported to occur in diabetes with poor glycaemic control.l3
In addition, heterogeneous results of unchanged,14 decreased15 or elevated16 activity of erythrocyte GPx have been reported. In this study, a significantly elevated GPx activity was observed in DM (21.87%) and DF subjects (20.94%), compared with the NC subjects. These findings accord with the work of Gupta and Chari16 in both types of diabetes, where an increase was also reported. The increase in the activity of this alternative antioxidant enzyme may constitute a compensatory mechanism to prevent further tissue damage in diabetic subjects.
Conclusion
Findings in this study are compatible with the hypothesis that persistent hyperglycaemia leads to increased production of oxidants (LPO and 8-OHdG) in diabetic subjects. The increase is more pronounced in subjects with DF. Increased oxidation subsequent to diabetic conditions induces an over-expression of GPx activity, suggesting a compensatory mechanism by the body to prevent further tissue damage in such subjects.
We gratefully acknowledge the financial support of Third World Organization for Women in Sciences (TWOWS) and the technical assistance of the Chemical Pathology Department of the National Health Laboratory Services (NHLS) of South Africa.
References
1. Sies H. What is oxidative stress? In: Keaney JF Jr, ed. Oxidative Stress and Vascular Disease. Boston: Kluwer Academic Publishers, 2000: 1-8. [ Links ]
2. Gumieniczek A, Hopkala H, Wojtowich Z, Nikolajuk J. Changes in antioxidant status of heart muscle tissue in experimental diabetes in rabbits. Acta Biochim Pol 2002; 49: 529-535. [ Links ]
3. Bonnefont-Rousselot D, Bastard JP, Jaudon MC, Delattre J. Consequences of the diabetic status on the oxidant/antioxidant balance. Diabetes Metab 2000; 26: 163-176. [ Links ]
4. Betteridge DJ. What is oxidative stress? Metabolism 2000; 49: 3-8. [ Links ]
5. Nouroouz-Zadeh J, Tajaddini-Sarmadi J, McCarthy S, Betteridge DJ, Wolff SP. Elevated levels of authentic plasma hydroperoxides in NIDDM. Diabetes 1995; 44: 1054-1058. [ Links ]
6. Toyokuni S, Tanaka T, Hattori Y, et al. Quantitative immunohistochemical determination of 8-hydroxy-2'-deoxyguanosine by monoclonal N45.1, its application to ferric nitrilotriacetate-induced renal carcinogenesis model. Lab Invest 1997; 76(3): 365-374. [ Links ]
7. Arthur JR, Boyne R. Superoxide dismutase and glutathione peroxidase activities in neutrophils from selenium deficient and copper deficient cattle. Life Sci 1985; 36: 1569-1575. [ Links ]
8. Paglia DE, Valentine WN. Glutathione peroxidase determination. J Lab Clin Med 1967; 70: 158-169. [ Links ]
9. Bhatia S, Shukla R, Madhu SV, Gambhir JK, Prabhu KM. Antioxidant status, lipid peroxidation and nitric oxide end products in patients of type 2 diabetes mellitus with nephropathy. Clin Biochem 2003; 36(7): 557-562. [ Links ]
10. Levy U, Zaltzber H, Ben-Amotz A, Kanter Y, Aviram M. B-carotene affects antioxidant status in non-insulin-dependent diabetes mellitus. Pathophysiol 1999; 6: 157-161. [ Links ]
11. Santini SA, Marra G, Giardina B, et al. Defective plasma antioxidant defenses and enhanced susceptibility to lipid peroxidation in uncomplicated IDDM. Diabetes 1997; 46(11): 1853-1858. [ Links ]
12. Dandona P, Thusu K, Cook S, et al. Oxidative damage to DNA in diabetes mellitus. Lancet 1996; 347(8999): 444-445. [ Links ]
13. Kelly FJ. Use of antioxidants in the prevention and treatment of disease. J Int Fed Clin Chem 1998; 10(1): 21-23. [ Links ]
14. Aydin A, Orhan H, Sayal A, Ozata M, Sahin G, Isimer A. Oxidative stress and nitric oxide related parameters in type II diabetes mellitus: effects of glycemic control. Clin Biochem 2001; 34: 65-70. [ Links ]
15. Kesavulu MM, Giri R, Kameswara RB, Apparao C. Lipid peroxidation and antioxidant enzyme levels in type 2 diabetics with microvascular complications. Diabetes Metab 2000; 26(5): 387-392. [ Links ]
16. Gupta MM, Chari S. Lipid peroxidation and antioxidant status in patients with diabetic retinopathy. Ind J Physiol Pharmacol 2005; 49(2): 187-192. [ Links ]
 Correspondence:
Correspondence:
K Mossanda
(kmossan@ul.ac.za)
Accepted 30 May 2008.














