Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.98 n.7 Pretoria Jul. 2008
FORUM
CLINICAL IMAGES
MRI features of disseminated 'drop metastases'
A Ahmed
Aadil Ahmed was a consultant radiologist at Chris Hani Baragwanath Hospital. He is currently doing a fellowship in Paediatric Radiology at the Children's Hospital at Westmead in Sydney, Australia
Cerebrospinal fluid (CSF) seeding, with the resultant formation of spinal intradural 'drop metastases', is a well-known mode of dissemination for many intracranial neoplasms.
This form of spread is encountered more frequently in paediatric patients than in adults.
This manner of tumour spread is most commonly observed in medulloblastoma. Up to one-third of patients with medulloblastoma will eventually disseminate tumour through the CSF, most often to the spine. The presence of metastases in the spinal canal indicates a particularly poor prognosis.1
Ependymomas, anaplastic gliomas, germinomas, choroid plexus tumours and pineal parenchymal tumours (pineoblastomas and pineocytomas) are other intracranial tumours that frequently deposit metastases in the spinal canal.
Retinoblastoma is not usually considered; however this mode of dissemination is well described, albeit less commonly encountered nowadays because of improved treatment options. The route of spread appears to be by direct extension into the optic nerve from the retina and into the meningeal spaces, or along the central retinal vessels to the subarachnoid space.2
Intradural metastases may also occur as a result of haematogenous spread from extracranial neoplasms, most commonly lung and breast adenocarcinoma, and from haematological malignancies.
The 2 cases presented here demonstrate extensive spinal canal drop metastases, occupying almost the entire canal from the craniocervical junction down to the lumbosacral spine. Both these patients had primary intracranial neoplasms.
Case 1
A 17-year-old girl presented with headache, diplopia and cerebellar signs of 4 months' duration, and inability to walk for a shorter period.
The brain magnetic resonance imaging (MRI) scan shows a large, predominantly solid tumour arising from the roof of the 4th ventricle, attenuating the 4th ventricle and causing obstructive hydrocephalus (Fig. 1). Postoperative histological examination confirmed a medulloblastoma.

The T2 sequence of the spine demonstrated lobulated iso- to hypointense masses occupying the thecal sac, with the spinal cord and CSF barely discernible in areas. After gadolinium (Gd) administration there was strong enhancement of the extensive intradural metastases, causing cord compression at multiple levels (Fig. 2). Multiple smaller metastatic nodules were present on the lumbar nerve roots.

Case 2
A 2-year-old girl had undergone surgery for a right-sided retinoblastoma before her current admission, which was for recent-onset myelopathy.
The surgery was performed at a peripheral hospital and the brain MRI scan showed the retained right optic nerve, which was probably the route of spread, as well as an intracranial leptomeningeal metastasis (Fig. 3).

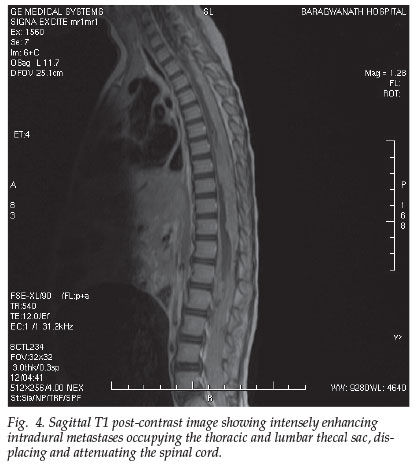
The MRI of the spine depicts the bulky extramedullary masses filling the entire thoracic and lumbar spinal canal, obliterating the thecal sac inferior to the conus (Fig. 4). These intradural masses were isointense to the cord on T1 and T2 and enhanced intensely post contrast, and in the thoracic region these metastases were seen to invade the cord.
The more frequently encountered patterns of drop metastases are multifocal, discrete nodules along the cord and nerve roots; or diffuse, thin, sheet-like coating of the cord.3,4 These patients therefore rarely have symptoms referable to the spine, and the metastases are more commonly picked up incidentally when their spines are screened in the presence of a primary intracranial tumour.
Myelography with or without computed tomography (CT) was previously the mode of investigation to search for CSF spread. The gold standard now is Gd-enhanced spinal MRI. This is superior to CT-myelography in paediatric cases of leptomeningeal metastases from intracranial tumours. MRI can be utilised to survey the entire spine and brain without the risks and radiation involved with myelography.5
The nodular drop metastases are typically hyperintense to CSF on proton density images, usually hypointense to the cord on T1, and hypointense to CSF on T2. After the administration of gadolinium, these extramedullary masses usually enhance markedly. These metastases may at times be very difficult to visualise without contrast administration.3 Nodules as small as 2 - 3 mm have been reported. Less commonly, leptomeningeal metastases may invade the spinal cord. Artefacts are common on MRI studies of the spine in the first few weeks after posterior fossa craniectomy, so it is essential to stage the tumour preoperatively.5
Intradural drop metastases are not an uncommon finding in a wide range of paediatric and adult primary intracranial neoplasms. The incidence appears to be increasing, as cancer patients are surviving for longer periods of time with improved treatment regimens. If these tumours are encountered or suspected, Gd-enhanced MRI of the entire neuraxis before surgery is essential.
1. Barkovich AJ. Pediatric Neuroimaging. 3rd ed. Philadelphia: Lippincott-Raven, 2000: 693. [ Links ]
2. Lipper MH, Kishore PR. Intraspinal metastases from retinoblastoma. Radiology 1979; 131: 161-163. [ Links ]
3. Zawadzki MB, Chen MZ, Moore KR, Salzman KL, Osborn AG. Pocket Radiologist Spine - Top 100 Diagnoses. 1st ed. Philadelphia: WB Saunders, 2002: 209-211. [ Links ]
4. Dahnert W. Radiology Review Manual 5th ed. Baltimore: Lippincott Williams & Wilkins, 2003. [ Links ]
5. St Amour TE, Hodges SC, Ross JS, et al. MRI of the Spine. 1st ed. New York: Raven Press, 1994. [ Links ]
 Correspondence:
Correspondence:
A Ahmed
(doca@webmail.co.za)














