Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.98 n.6 Pretoria Jun. 2008
ORIGINAL ARTICLES
Pneumococcal conjugate vaccine - a health priority
Heather J ZarI; Shabir A MadhiII
IMB BCh, FAAP, BC Paed Pulmonology, PhD. Department of Paediatric Pulmonology and School of Child and Adolescent Health, Red Cross War Memorial Children's Hospital and University of Cape Town
IIMB BCh, MMed (Paed), FCPaeds (SA), PhD. Medical Research Council: Respiratory and Meningeal Pathogens Research Unit, and Department of Science and Technology/National Research Foundation: Vaccine Preventable Diseases, University of the Witwatersrand
ABSTRACT
Pneumonia is a major cause of childhood mortality and morbidity. Streptococcus pneumoniae is the most important bacterial pathogen causing pneumonia in children. The HIV epidemic has increased the burden and severity of childhood pneumococcal pneumonia and invasive disease fortyfold.
Pneumococcal conjugate vaccine (PCV) is a highly effective intervention to reduce invasive pneumococcal disease and pneumonia. Studies evaluating a 9-valent PCV in South Africa and The Gambia reported a 72 - 77% reduction in vaccine-serotype-specific invasive disease in vaccinated children. As many of the pneumococcal serotypes associated with antibiotic resistance are included in PCV, vaccination has also been associated with a reduction in antimicrobial-resistant invasive disease. PCV may also reduce childhood mortality, especially in places with limited access to health care, as shown in Gambian study in which PCV reduced childhood mortality by 16%. In addition to the direct effects of PCV, there is a substantial reduction in disease burden through indirect protection of non-vaccinated populations.
PCV is immunogenic in HIV-infected children and provides protection against invasive disease or pneumonia in a substantial number of children. Although the efficacy of PCV for prevention of invasive disease or pneumonia is lower in HIV-infected compared to -uninfected children, the overall burden of disease prevented is much greater in HIV-infected children because of the higher burden of pneumococcal disease in these children. Consequently, vaccine-preventable invasive disease is almost 60 times higher in HIV-infected compared to -uninfected children, while the reduction in pneumonia in HIV-infected children is 15 times greater. However, the long-term efficacy of PCV wanes in HIV-infected children who are not taking antiretroviral therapy, and booster doses are probably indicated.
Although there is concern about the potential for replacement disease due to non-vaccine serotypes, a substantial and sustained reduction in invasive disease has occurred overall in populations with widespread childhood immunisation. Routine childhood immunisation is now the standard of care in most developed countries. However, PCV is much less accessible to children in developing countries due to cost and availability. Cost-effectiveness analysis indicates that use of PCV is potentially highly cost-effective, at tiered pricing, even in very low-income countries.
Widespread availability and vaccination with PCV is urgently needed for all children under 2 years of age in South Africa. In addition, the use of PCV for all HIV-infected children under 9 years should be prioritised.
Pneumonia is a major cause of childhood mortality and morbidity globally, resulting in 2 million deaths a year.1-3 Pneumonia is the direct cause of approximately 20% of all childhood deaths. In addition, for every death directly attributable to pneumonia, there are 2 to 3 additional deaths due to comorbid conditions such as malnutrition or measles.1 The overwhelming burden of childhood pneumonia and associated deaths is in developing countries, 1-3 in many of which the HIV epidemic has increased the incidence, severity and complexity of childhood pneumonia.
In South Africa, childhood pneumonia has been a major cause of mortality for decades. Despite improvements in access to care, greater health equity and economic growth, the under-5 mortality rate in South Africa has increased to 66 per 1 000, representing an increase of 1.3% from 1995 to 1999 and of 1.6% from 2000 to 2003.4 This is in contrast to the fourth Millennium Development Goal which aims to reduce childhood mortality by two-thirds by 2015. In South Africa this would mean a reduction in under-5 mortality from 60 per 1 000 in 1995 to 20 per 1 000 by 2015.5
The burden of pneumococcal pneumonia
Bacterial infections are the major preventable cause of hospitalisation and death from pneumonia in children in developing countries. Among children who died of respiratory disease in Zambia, bacterial pneumonia was the most commonly identified cause of death in 41% of HIV-infected and 50% of HIV-uninfected children. Co-existing other non-bacterial respiratory pathogens were also frequently identified.6Streptococcus pneumoniae is the most important bacterial pathogen causing pneumonia in HIV- infected and -uninfected children.7-9
S. pneumoniae causes between 1 and 4 million episodes of pneumonia in Africa each year.10 Bacteraemia occurring with pneumonia is a severe manifestation of disease. The incidence of pneumococcal bacteraemia or invasive pneumococcal disease (IPD) is 9 - 43 times greater in HIV-infected than in HIV-uninfected children.11,12 Thus, the incidence of bacteraemic pneumococcal pneumonia has increased exponentially as the HIV epidemic has escalated in South Africa. For example, the incidence of IPD has increased from 200 per 100 000 children per year in 1987 (before the onset of the HIV epidemic) to 380 by 1996 - 1997, and to more than 500 cases per 100 000 children in 200513 (unpublished data for 2005 - S A Madhi.) Importantly, 75% of serious IPD in South African children occurs in the 5 - 6% of the childhood population who are HIV-infected.11
The minimum burden of pneumococcal pneumonia in South African children can be estimated by using pneumococcal conjugate vaccine (PCV) as a probe. In a South African trial using a 9-valent PCV, the burden of pneumonia prevented by vaccine in HIV-infected children (2 573 cases prevented per 100 000 child-years) was almost ten times that in HIV-uninfected (267 cases prevented per 100 000 child-years).14 Globally, pneumococcal disease is the leading cause of vaccine-preventable death in children <5 years of age. The World Health Organization (WHO) has estimated that, at current levels of global coverage for 3 doses of diptheria-tetanus-pertussis (DTP3) vaccine in the EPI immunisation programme, as many as 430 000 deaths could be prevented annually by introducing PCV into developing countries. If global vaccination coverage for DTP3 increased to 90%, almost 900 000 childhood deaths could be prevented.15
Childhood pneumonia is frequently caused by mixed infections such as bacterial-viral or bacterial-mycobacterial pathogens, resulting in more severe illness.9 As evidence of this, children who received PCV were less likely to develop severe viral-associated pneumonia requiring hospitalisation, with a 32% reduction in admissions for viral-associated pneumonia in immunised children.16 Moreover, mortality rates from pneumonia increase exponentially with increasing numbers of pathogens. Children with polymicrobial pneumonia have a tenfold greater risk of dying than children in whom only a single organism is identified.9
Prevention of pneumococcal pneumonia in children using conjugate vaccine
The development of PCV has been a major advance in preventing pneumoccocal pneumonia. Unlike the polysaccharide pneumococcal vaccines, which are poorly immunogenic in children under 5 years of age, PCV is immunogenic, safe and effective in children immunised from 6 weeks of age onward. Globally, about 20 serotypes are associated with more than 80% of IPD, with the 13 most common serotypes causing at least 70 - 75% of invasive disease in children.15 The only commercially available PCV includes 7 serotypes (PCV7) which cause almost 70% of all IPD in South African children.17 Changing from the 7-valent to a 10-valent vaccine would increase the proportion of serotypes covered from 67% to 81% in African, and to 85% in South African, children.15,17 Further improvements in serotype coverage may occur with added serotypes being included in future formulations.
Efficacy of pneumococcal conjugate vaccine
Pneumococcal conjugate vaccine is highly effective against serotype-specific IPD. An evaluation of PCV7 in the USA showed a 94% reduction in IPD from vaccine strains.18 Evaluation of a 9-valent PCV in South Africa and The Gambia demonstrated a 72 - 77% reduction in vaccines-serotype (VT)-specific IPD among vaccinated children (Table I).19,20 The efficacy against IPD regardless of pneumococcal serotype was 45% in Gambia.20 Although the efficacy against VT-IPD was lower in HIV-infected (65%) than HIV-uninfected children (83%) in South Africa, the absolute burden of IPD prevented by vaccination was 18 times greater in HIV-infected children (570 v. 32 per 100 000 children vaccinated, respectively).19
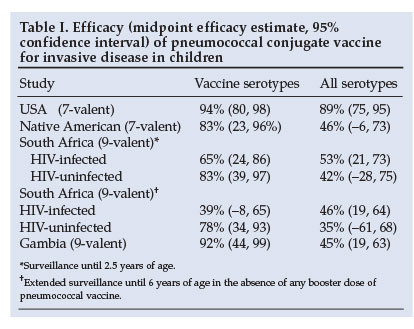
Further evidence of the efficacy of PCV comes from the impact on IPD in high-risk paediatric populations. In Alaskan children, IPD rates have fallen dramatically since the introduction of PCV7 in 2001.21 In Australian Aboriginal children under 2 years of age, 3 doses of PCV7 plus a dose of 23-valent vaccine at 18 months reduced VT-IPD from 118 to 43/100 000/year, even though the coverage was only about 54%.22 The rate of non-vaccine serotype IPD remained constant at 31/100 000/year.
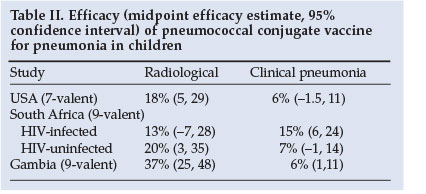
The incidence of radiologically confirmed pneumonia is also reduced by vaccination, varying from 13% to 37% (Table II).19,20,23 Based on WHO-defined radiological pneumonia, the adjusted efficacy for prevention of a first episode of pneumonia was 25% with PCV7 for intent-to-treat in the USA.24 However, the sensitivity of chest radiographs in detecting the burden of pneumococcal pneumonia (as defined by WHO criteria) may underestimate the burden of disease by as much as 63%. The true burden of pneumococcal pneumonia prevented by vaccination may therefore be much greater than that detected by chest radiographs. As evidence of this, a North American study reported a 61% reduction in all-cause pneumonia admissions in children <2 years associated with routine use of PCV7 compared with the pre-vaccination era.25
As many of the pneumococcal serotypes associated with antibiotic resistance are included in PCV7, vaccination with PCV has also been associated with a reduction in antimicrobial-resistant IPD. After the introduction of PCV7, the USA experienced an 80% decline in IPD caused by penicillin-resistant strains in young children.26 In the South African PCV9 study, a reduction in IPD from penicillin and trimethoprim-sulfamethoxazole resistant strains of 67% and 56% respectively was found in immunised children.19 Given the increasing prevalence of drug-resistant pneumococci, a vaccine that reduces IPD and carriage of potentially drug-resistant serotypes is of major public health value.
The impact of PCV on childhood mortality depends on the context and availability of health care. PCV9 reduced all-cause childhood mortality by 16% in Gambia, in which children from an impoverished rural population with limited access to health care were enrolled.20 In contrast, in the South African study in the urban setting of Soweto, where children have free access to primary and hospital health care, no effect on childhood mortality was evident.27 Therefore the benefit of vaccination against mortality will be greatest among vulnerable populations with limited access to health care.
In addition to the direct effects of PCV, there is a substantial reduction in disease burden through indirect protection of non-vaccinated populations. Children (who are the primary means of transmission of pneumococci) are protected against colonisation with vaccine serotypes after PCV immunisation.28 Consequently, reduced colonisation by vaccine serotypes in vaccinated children interrupts the transmission of these serotypes and reduces the risk of other susceptible individuals in the community becoming colonised. As evidence of this indirect protection, the rate of VT-IPD in the USA has fallen across age groups. The reduction in IPD has been most marked in children <5 years of age (who are the target group for immunisation) as well as in the age groups of 40 - 65 years and >65 years (who are not immunised, but have reduced rates of disease via the indirect benefit of reduced transmission).29 Consequently in the USA, universal immunisation of children with PCV7 has been estimated to directly reduce the number of cases of IPD by approximately 9 000 among vaccinated children, while indirect protection resulted in a further 20 000 cases of IPD being prevented per year.29
Efficacy in HIV-infected children
PCV is immunogenic in HIV-infected children. HIV-infected infants on highly active antiretroviral therapy (HAART) have similar quantitative antibody responses to PCV, compared with HIV-uninfected children.30 However, HIV-infected children not on HAART have lower quantitative antibody responses to PCV9 and impaired antibody functionality against some serotypes, compared with HIV-uninfected children.31
PCV provides protection against IPD and pneumonia in a substantial number of HIV-infected children.12,14,19 In South Africa, the serotypes included in PCV9 were associated with 83 - 91% of invasive isolates among HIV-infected children.12 The efficacy of PCV9 against pneumonia varies, depending on the criteria used for defining 'pneumonia'. For example, the median reduction in radiologically confirmed pneumonia in HIV-infected children was 13% (95% confidence interval -7 to +28), in clinical pneumonia was 15% (6 - 24), and in bacteraemic pneumonia was 65% (24 - 86).14 However, the vaccine-attributable rate reduction was much greater for clinical pneumonia than bacteraemic disease (2 573 cases prevented per 100 000 child-years compared with 483 episodes of bacteraemic pneumococcal pneumonia prevented per 100 000 child-years), because the sensitivity of blood cultures in diagnosing pneumococcal pneumonia is only 3 - 20%.14
Although the efficacy of PCV9 for prevention of IPD and pneumonia was lower in HIV-infected compared with -uninfected children (Tables I and II), the overall burden of disease that is prevented is much greater, mainly because of the higher underlying burden of pneumococcal disease in HIV-infected children.14 Consequently, the overall vaccine-preventable disease of IPD was almost 60 times higher in HIV-infected compared with -uninfected children, while the reduction in pneumonia in HIV-infected children was 15 times greater at 6 years of follow-up.
The long-term efficacy of PCV wanes in HIV-infected children. In the context of limited access to HAART, vaccine efficacy against IPD declined in HIV-infected children from 65% (2.5 years post-vaccination) to 39% by 6 years of age in the absence of a booster dose (Table I). In contrast, efficacy was maintained in HIV-uninfected subjects.27 However, at 6 years' follow-up, the vaccine-attributable rate reduction in IPD was still 59 times greater in HIV-infected children compared with HIV-uninfected children (2 250 v. 38 cases prevented per 100 000 child-years). The need and benefit of intermittent booster doses of PCV requires further evaluation in HIV-infected children.
The potential indirect benefit of PCV to HIV-infected adults through immunisation of children should also not be underestimated. Pneumococcal disease, especially that caused by serotypes included in PCV7,32 is a major cause of mortality and morbidity in HIV-infected adults and in the elderly. The potential benefit of childhood immunisation on the development of herd immunity in populations with a high HIV prevalence needs further study.
Cost efficacy
A cost-efficacy analysis of 3 vaccine doses in infants, done in 72 countries eligible for support from Global Alliance for Vaccines and Immunization (GAVI), found that vaccines could prevent 262 000 deaths per year (7% of children aged 3 - 29 months in the countries studied).33 This was estimated to save 8.34 million disability-adjusted life-years (DALYs) yearly. At $5 per dose, vaccination would cost $838 million (a cost of $100 per DALY averted) and is highly cost-effective.33 However, South Africa does not qualify for GAVI funding and PCV is not included in the EPI programme because of cost-constraints related to its market price (approximately $50 per dose). A vaccine that will especially benefit immunocompromised children, or children with poor access to health care facilities, therefore remains inaccessible to the children who are most susceptible to developing severe disease in this country.
Replacement disease
Replacement with serotypes that are not contained in a vaccine is a concern for development of disease and nasopharyngeal carriage. However, most serious illness is caused by a relatively small number of serotypes.29 Universal immunisation of infants with PCV7 reduces the carriage of vaccine strains, which is offset by an increase in carriage of non-vaccine pneumococci.28 Data suggest an increasing emergence of pneumococcal disease from non-vaccine serotypes in immunised children.21,34,35 Although this has predominantly been due to a single serotype 19A (an important cause of IPD before the introduction of PCV in the USA), the absolute magnitude of increased disease in the USA because of non-vaccine serotypes has been minimal, relative to the burden of disease prevented by vaccination.29 Conversely, the burden of non-vaccine serotype disease has increased 3 years after the introduction of PCV in Alaskan infants, attenuating the reduction in the burden of IPD by PCV. However, the overall incidence of IPD is still significantly lower compared with the incidence of IPD before the introduction of PCV.21 While replacement disease due to non-vaccine serotypes may be dealt with by broadening the number of serotypes included in PCV, continued monitoring of the effect of PCV on the epidemiology of IPD is necessary,
Recommendations
The use of PCV should be complementary to other pneumonia-control measures, including case management and the reduction of exposure to known risk factors such as malnutrition, biomass fuel exposure, tobacco smoke and HIV infection. WHO recommends that the inclusion of PCV7 vaccine should be a priority in national immunisation programmes, particularly in countries where mortality among children under 5 years is more than 50 per 1 000 live births or where more than 50 000 children die annually.15 Furthermore, WHO recommends that countries with a high prevalence of HIV prioritise the introduction of PCV7.15
Widespread availability and vaccination is urgently needed for ALL children under 2 years of age in South Africa. The introduction of PCV for all HIV-infected children less than 9 years of age (the upper age limit for which the vaccine is approved) should be prioritised. Children living in poorly resourced areas or where access to health care is limited, stand to benefit the most in terms of potential reduction in pneumococcal-associated morbidity and mortality.
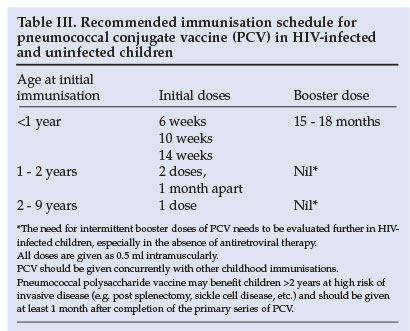
The recommended schedule for vaccination in infants is 3 doses of PCV given intramuscularly at least 4 weeks apart, beginning at 6 weeks of age concurrently with the other routine childhood vaccines, followed by a booster dose of PCV at 15 -18 months of age, which may be especially important in HIV-infected children (Table III). Children immunised between 12 and 24 months of age should receive at least 2 doses 1 month apart; and children aged 2 - 9 years of age require a single dose of PCV (Table III). Additional booster doses may be necessary in HIV-infected children in whom immunity wanes and loss of protection against invasive disease in the absence of a booster dose of PCV occurs.27
Conflict of interest. Dr S Madhi has received research support and serves on the speakers' bureau for Wyeth Vaccines and Pediatrics.
References
1. Williams BG, Gouws E, Boschi-Pinto C, Bryce J, Dye C. Estimates of world-wide distribution of child deaths from acute respiratory infections. Lancet Infect Dis 2002; 2(1): 25-32. [ Links ]
2. Bryce J, Boschi-Pinto C, Shibuya K, Black RE. WHO estimates of the causes of death in children. Lancet 2005; 365(9465): 1147-1152. [ Links ]
3. Black RE, Morris SS, Bryce J. Where and why are 10 million children dying every year? Lancet 2003; 361(9376): 2226-2234. [ Links ]
4. UNICEF. State of the World's Children. Statistical Tables. New York: UNICEF, 2005: 142-145. [ Links ]
5. Bryce J, Victora CG. Child survival: countdown to 2015. Lancet 2005; 365(9478): 2153-2154. [ Links ]
6. Chintu C, Mudenda V, Lucas S et al. Lung diseases at necropsy in African children dying from respiratory illnesses: a descriptive necropsy study. Lancet 2002; 360(9338): 985-990. [ Links ]
7. Zar HJ, Hanslo D, Tannenbaum E et al. Aetiology and outcome of pneumonia in human immunodeficiency virus-infected children hospitalized in South Africa. Acta Paediatr 2001; 90(2): 119-125. [ Links ]
8. Madhi SA, Petersen K, Madhi A, Khoosal M, Klugman KP. Increased disease burden and antibiotic resistance of bacteria causing severe community-acquired lower respiratory tract infections in human immunodeficiency virus type 1-infected children. Clin Infect Dis 2000; 31(1): 170-176. [ Links ]
9. McNally LM, Jeena PM, Gajee K, et al. Effect of age, polymicrobial disease, and maternal HIV status on treatment response and cause of severe pneumonia in South African children: a prospective descriptive study. Lancet 2007; 369(9571): 1440-1451. [ Links ]
10. Scott JA. The preventable burden of pneumococcal disease in the developing world. Vaccine 2007; 25(13): 2398-2405. [ Links ]
11. Madhi SA, Petersen K, Madhi A, Wasas A, Klugman KP. Impact of human immunodeficiency virus type 1 on the disease spectrum of Streptococcus pneumoniae in South African children. Pediatr Infect Dis J 2000; 19(12): 1141-1147. [ Links ]
12. Bliss SJ, O'Brien KL, Janoff EN, et al. The evidence for using conjugate vaccines to protect HIV-infected children against pneumococcal disease. Lancet Infect Dis 2008; 8(1): 67-80. [ Links ]
13. Karstaedt AS, Khoosal M, Crewe-Brown HH. Pneumococcal bacteremia during a decade in children in Soweto, South Africa. Pediatr Infect Dis J 2000; 19(5): 454-457. [ Links ]
14. Madhi SA, Kuwanda L, Cutland C, Klugman KP. The impact of a 9-valent pneumococcal conjugate vaccine on the public health burden of pneumonia in HIV-infected and -uninfected children. Clin Infect Dis 2005; 40(10): 1511-1518. [ Links ]
15. Pneumococcal conjugate vaccine for childhood immunization - WHO position paper. Wkly Epidemiol Rec 2007; 82(12): 93-104. [ Links ]
16. Madhi SA, Klugman KP. A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat Med 2004; 10(8): 811-813. [ Links ]
17. von Gottberg A, de Gouveia L, Quan V, et al. Vaccine-preventable invasive pneumococcal disease, South Africa, S Afr J Epidemiol Infect 2007; 22: 95 (abstract 101). [ Links ]
18. Black S, Shinefield H, Fireman B, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J 2000; 19(3): 187-195. [ Links ]
19. Klugman KP, Madhi SA, Huebner RE, Kohberger R, Mbelle N, Pierce N. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med 2003; 349(14): 1341-1348. [ Links ]
20. Cutts FT, Zaman SM, Enwere G, et al. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-blind, placebo-controlled trial. Lancet 2005; 365(9465): 1139-1146. [ Links ]
21. Singleton RJ, Hennessy TW, Bulkow LR, et al. Invasive pneumococcal disease caused by nonvaccine serotypes among Alaskan native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA 2007; 297(16): 1784-1792. [ Links ]
22. Giele C, Moore H, Bayley K, et al. Has the seven-valent pneumococcal conjugate vaccine had an impact on invasive pneumococcal disease in Western Australia? Vaccine 2007; 25(13): 2379-2384. [ Links ]
23. Black SB, Shinefield HR, Ling S, et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr Infect Dis J 2002; 21(9): 810-815. [ Links ]
24. Hansen J, Black S, Shinefield H, et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than 5 years of age for prevention of pneumonia: updated analysis using World Health Organization standardized interpretation of chest radiographs. Pediatr Infect Dis J 2006; 25(9): 779-781. [ Links ]
25. Grijalva CG, Nuorti JP, Arbogast PG, Martin SW, Edwards KM, Griffin MR. Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: a time-series analysis. Lancet 2007; 369(9568): 1179-1186. [ Links ]
26. Kyaw MH, Lynfield R, Schaffner W, et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med 2006; 354(14):1455-1463. [ Links ]
27. Madhi SA, Adrian P, Kuwanda L, et al. Long-term immunogenicity and efficacy of a 9-valent conjugate pneumococcal vaccine in human immunodeficient virus infected and non-infected children in the absence of a booster dose of vaccine. Vaccine 2007; 25(13): 2451-2457. [ Links ]
28. Klugman KP. Efficacy of pneumococcal conjugate vaccines and their effect on carriage and antimicrobial resistance. Lancet Infect Dis 2001; 1(2): 85-91. [ Links ]
29. Reingold A, Hadler J, Farley MM, et al. Direct and indirect effects of routine vaccination of children with 7-valent pneumococcal conjugate vaccine on incidence of invasive pneumococcal disease-United States, 1998-2003. MMWR Morb Mortal Wkly Rep 2005; 54(36): 893-897. [ Links ]
30. Nachman S, Kim S, King J, et al. Safety and immunogenicity of a heptavalent pneumococcal conjugate vaccine in infants with human immunodeficiency virus type 1 infection. Pediatrics 2003; 112(1 Pt 1): 66-73. [ Links ]
31. Madhi SA, Kuwanda L, Cutland C, Holm A, Kayhty H, Klugman KP. Quantitative and qualitative antibody response to pneumococcal conjugate vaccine among African human immunodeficiency virus-infected and uninfected children. Pediatr Infect Dis J 2005; 24(5):410-416. [ Links ]
32. Buie KA, Klugman KP, von Gottberg A, et al. Gender as a risk factor for both antibiotic resistance and infection with pediatric serogroups/serotypes, in HIV-infected and -uninfected adults with pneumococcal bacteremia. J Infect Dis 2004; 189(11): 1996-2000. [ Links ]
33. Sinha A, Levine O, Knoll MD, Muhib F, Lieu TA. Cost-effectiveness of pneumococcal conjugate vaccination in the prevention of child mortality: an international economic analysis. Lancet 2007; 369(9559): 389-396. [ Links ]
34. Pelton SI, Huot H, Finkelstein JA, et al. Emergence of 19A as virulent and multidrug resistant Pneumococcus in Massachusetts following universal immunization of infants with pneumococcal conjugate vaccine. Pediatr Infect Dis J 2007; 26(6): 468-472. [ Links ]
35. Barricarte A, Castilla J, Gil-Setas A, et al. Effectiveness of the 7-valent pneumococcal conjugate vaccine: a population-based case-control study. Clin Infect Dis 2007; 44(11): 1436-1441. [ Links ]
 Correspondence:
Correspondence:
Prof H Zar
(heather.zar@uct.ac.za)
Accepted 23 January 2008.














