Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SAMJ: South African Medical Journal
On-line version ISSN 2078-5135
Print version ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.98 n.6 Pretoria Jun. 2008
FORUM
ISSUES IN PUBLIC HEALTH
A review of the prevention of mother-to-child transmission programme of the Western Cape provincial government, 2003 - 2004
Beverly DraperI; Fareed AbdullahII
IDr Draper was Programme Manager: Child Health Service of the Children's Institute (based at the University of Cape Town) at the time of acceptance of this article. She now works at the Knowledge Translation Unit of the UCT Lung Institute. The article came from her experience as the provincial co-ordinator of the PMTCT programme of the Department of Health of the Western Cape during 2003 and 2004
IIFareed Abdullah is Director: Technical Support of the International HIV/AIDS Alliance, Brighton, UK, an international NGO which was set up in 1993 by a consortium of international donors
By 2006, nearly 25 million adults and children in sub-Saharan Africa were living with HIV.1 The national antenatal seroprevalence survey in South Africa in 2005 showed that 30.2% of pregnant women were HIV positive,2 which implies enormous potential for mother-to-child transmission (MTCT) of the virus. MTCT of HIV remains the most significant source of HIV infection in children; therefore, this mode of infection constitutes an important aspect of the overall HIV/AIDS epidemic in South Africa. Clinical trials testing the effectiveness of administering antiretrovirals to pregnant women before delivery, and to their newborns, have demonstrated that effective interventions exist that can dramatically reduce the rate of transmission from HIV-positive mothers to their infants.3 In 2001, the United Nations General Assembly Special Session on HIV/AIDS called for a reduction of the proportion of infants infected with HIV by 20% by 2005, and by 50% by 2010.4 The challenge is to implement effective, affordable, safe and acceptable interventions, at this scale, in resource-constrained settings.5
The Western Cape province was the first to launch prevention of mother-to-child transmission (PMTCT) programmes in South Africa. In January 1999, the sub-district of Khayelitsha was selected as a pilot site to initiate a PMTCT programme in the province, offering HIV counselling and testing, zidovudine at 34 weeks' gestation and during labour, formula-feeding and infant HIV testing. By 2001, the programme was extended to a further 5 sites, by which time nevirapine was available and used as a single-dose regimen for mothers and infants.6 By early 2003, the programme had been 'rolled out' to all maternal and infant service sites throughout the province as an essentially nurse-driven service. In July 2003, research results presented at the International AIDS Conference in Barcelona demonstrated that dual drug therapy including short-course zidovudine and single-dose nevirapine to both HIV-positive mothers and their babies could significantly reduce the transmission rate,7 and the provincial PMTCT protocol was revised accordingly, as well as CD4 testing for all HIV-positive pregnant women and polymerase chain reaction (PCR) HIV testing of their infants. 8 The history of the development of the provincial PMTCT protocol, and changes in the protocol, is set out in Table I.
An HIV antiretroviral treatment (ART) programme was initiated in the Western Cape province in 2004 in parallel with the revision of the PMTCT programme. Routine CD4 count testing facilitated the referral and initiation of highly active antiretroviral therapy (HAART) during the antenatal period for women who had high viral loads and were at high risk of transmitting HIV to their babies.
The aim of this paper is to review the changes made to the Western Cape's PMTCT protocol and its impact on programme outputs and outcomes in the provincial health system. The objectives are to describe (i) the programme indicators before and after the changes made to the PMTCT protocol; (ii) the introduction of routine CD4 count testing into the programme; and (iii) the interface of the PMTCT programme with the introduction of the ART programme.
Methods
Setting
The data presented in this paper were based on routine information collected during 2003 and 2004. The Western Cape province currently comprises 6 districts - 1 urban and 5 rural. There are differences in the structure of maternity services between urban and rural districts. Deliveries in rural districts are managed at district hospitals, while in the Cape Town metropole 42% of all deliveries are conducted at midwife obstetric units (MOUs) at primary care level; only complications are referred to the appropriate hospital.
The information system for PMTCT
Registers to collect specific data for the PMTCT programme were developed for antenatal clinics, labour wards and baby clinics. Sub-district HIV co-ordinators were in place to collect and validate operational data. This information was reviewed by district co-ordinators before being submitted to the provincial office. Indicators that are required from the data are listed in Table II.
CD4 counts were performed on all antenatal patients who tested HIV positive, but were not captured as routine data. However, statistics were collected by the National Health Laboratory Services (NHLS) for some sub-districts or facilities from which such specimens were submitted, to establish the proportion of CD4 counts that were below 200 in these HIV-positive women in the PMTCT programme. The purpose of the analysis was to establish what proportion of HIV-positive pregnant women would require assessment for ART as it became available.
Results
In broad terms, a PMTCT programme should reveal the number of pregnant women who are identified as HIV infected, and the success of the interventions to reduce transmission. Further, the programme should identify HIV-positive women who are at risk of advanced disease and ensure appropriate referral for management.
1. Uptake of HIV testing by pregnant women
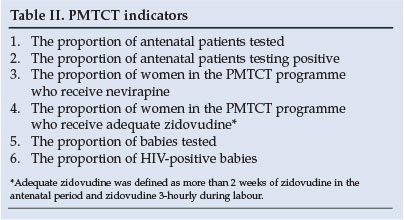
The proportion of women tested increased from 86% in 2003 to 96% in 2004. The total number of women tested in the PMTCT programme increased from 65 169 in 2003 to 82 747 in 2004 -an increase of 21% in the total number of women tested. The urban and rural distribution is shown in Fig. 1.
2. Number of women testing HIV positive
A total of 8 093 women (12.4%) tested HIV positive in 2003, and 9 721 (11.8%) in 2004. For both years, the proportion was highest in the metropole district. The HIV-positive figure for some metropole sub-districts exceeded 20%.
3. Antiretroviral uptake
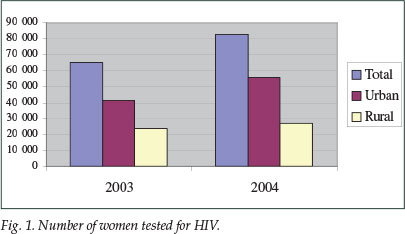
Nevirapine as a single dose to mothers and infants was recorded in 2003 as a take-home dose, and in 2004 as a facility-based dose. The proportion of nevirapine uptake did not significantly increase from 2003 to 2004, and remained at 60%. There was, however, a marked shift in practice, consistent with the revised policy, from the take-home dose that was self-administered to the facility-based dose administered by maternity staff (Fig. 2). While in 2003 the nevirapine dose was predominantly a take-home dose (69%), by 2004 most women were receiving nevirapine on admission in labour (93%).
The reporting rate for adequate zidovudine in 2004 was 94%, which indicates the proportion of HIV-positive women for whom information was actually recorded regarding administration of zidovudine. Of these, it was recorded that 71% received adequate zidovudine (this was defined as at least 2 weeks of antenatal zidovudine). The coverage for nevirapine and zidovudine was the same for urban and rural districts.
4. Infant testing and transmission
Testing of infants at 9 months up to 2003 had been a challenge in all the districts, with considerable loss to follow-up. The change in data collection and introduction of new baby registers was found to be slow in the rural districts, and conversion to a system of testing babies according to monthly birth cohorts took a number of months to implement. The proportion of infants tested was measured as the number of infants tested as a proportion of the number of infants born to HIV-positive women. The data on infant testing from the rural districts in 2004 could not be considered sufficiently valid to indicate actual rates of MTCT. The best data available that may indicate the success of the programme are from the metropole district. In 2003, the proportion of infants tested in the metropole district was 8%, and 17.5% of these children tested HIV positive. In 2004, with the introduction of PCR testing at 14 weeks of age, 80% of infants in the metropole were offered HIV testing; among this group, there was a 98% acceptance rate. Seven per cent of these infants tested HIV positive. In 8 of the 9 urban sub-districts, less than 10% of infants tested HIV positive. In addition, 72% demonstrated good formula-feeding compliance.
5. CD4 counts
Owing to logistical problems, data were available for only part of 2004 from the Groote Schuur Hospital and Tygerberg Hospital laboratories in the Western Cape, where CD4 counts for the PMTCT programme were performed. There was a steady increase in the number of monthly CD4 counts that were submitted for women in the PMTCT programme, and CD4 counts were done on 51% of PMTCT patients during the second half of 2004. Of these, an average of 19.8% were below 200 cells/µl.
6. Referrals from the PMTCT programme to ARV clinics
Once women were being identified as having low CD4 counts by routine CD4 testing early in pregnancy, it was important to identify what steps were required for appropriate management of these patients. HIV-positive women with CD4 counts <200 were considered to be patients at risk, both obstetrically and for increased vertical HIV transmission. Protocols were developed during 2004 to efficiently facilitate the referral of PMTCT patients with low CD4 counts to ARV sites. Two weekly satellite ARV clinics were set up in the MOUs in one urban sub-district with an HIV prevalence of 27%. Between December 2004 and November 2005, 130 patients had been seen at one of these MOU ARV clinics; 93% of these had been referred before 29 weeks' gestation and were able to be initiated on ART in sufficient time before delivery.
Discussion
The results presented here are based on data from the standard reporting on the PMTCT programme within the provincial health system which, in the absence of formal sampling, give an indication of how the PMTCT programme is functioning in the province, and where further improvements may be necessary. It must be conceded that the results for 2003 and 2004 were limited by the constraints of change and the introduction of new information that needed to be collected. Therefore, these results should be viewed within this context of change and operational challenges.
High uptake of HIV testing suggests an improvement on previous years in counselling in the programme.9 An increase in the proportion of women tested could have been partly the result of retraining of counsellors in the revised protocols. The increase in absolute numbers of women who tested HIV positive in 2004 compared with 2003 may be significant for the delivery of health services. It may be hypothesised that this increase in numbers may be attributed to a combination of migration of pregnant women from other provinces to deliver in the Western Cape,10 a higher testing rate in certain districts, and natural population increase. The PMTCT HIV prevalence per district was similar to the HIV prevalence results in the province during the same years.11
An average nevirapine uptake exceeding 80% would be more desirable than that of around 60% recorded for both years. The question must be asked whether the remaining 40% received any or no nevirapine; there are no data to indicate whether this shortfall of uptake is due to failure to administer the drug, or failure to record administration. Nevirapine resistance in the post-gestational period must be considered as an important issue to be addressed.12 The zidovudine uptake from April 2004 appeared steady, with little variation between the districts, although one might have expected a variation in the administration of zidovudine between rural and urban districts, and that women in rural areas might not have been adequately covered as the result of more limited access to antenatal services. Although the data on drug uptake are limited in scope, they are helpful in determining the uptake of dual drug administration and acquiring knowledge of the operational success of the revised programme.13
A major information gap remains: an accurate picture of the serostatus of infants born to women in the programme who subsequently tested HIV positive, which has proved to be a challenge in most developing countries.14 The improvement in testing and transmission rates of infants as recorded in the metropole district is the most significant improvement from 2003 to 2004. PCR testing that is conducted much earlier in infancy succeeds in capturing a larger proportion of babies born to mothers who have gone through the programme, and appears to have been easily adopted as a replacement to the rapid test. The high formula-feeding compliance of 72% recorded in the metropole further supports the infant testing results. Although formula compliance is not required as an official indicator, it may be seen to add validity to the transmission results.
The proportion of women on whom a CD4 count was performed was not recorded as routine data, and laboratory data for the first half of 2004 were specifically accessed to assess the progress of CD4 testing. Operationally, CD4 testing was successfully introduced into the Western Cape PMTCT programme and proved to be especially useful in areas of high HIV prevalence to identify high-risk individuals. Routine recording of the proportion of HIV-positive women who undergo CD4 testing should be recommended as a useful indicator for a PMTCT programme. In addition, recording of CD4 count results and the successful referral of women requiring HAART can supply valuable evidence for service delivery. This would allow the necessary expansion of access to HAART that is demanded for women accessing PMTCT services.15 Efficient management of these patients through early identification, referral and appropriate management with HAART should ensure a further decline in the transmission rate.16
Conclusion
New research has shown improved efficacy with a regimen of single-dose nevirapine combined with a short course of zidovudine to both mothers and infants. It has also become apparent that HIV-positive pregnant women who need ART for their own health should receive it during pregnancy. The provincial PMTCT programme has made significant strides in this direction and has shown that it is possible to move forward with new approaches to prevent MTCT in South Africa.
1. UNAIDS/WHO AIDS. Epidemic Update. Geneva: UNAIDS, December 2006. [ Links ]
2. Department of Health. National HIV and Syphilis Prevalence Survey 2005. Pretoria: Department of Health, 2005. [ Links ]
3. McIntyre J. Approaches to reducing vertical transmission of HIV from mothers to infants. Journal of Continuing Medical Education 2000; 18: 307-311. [ Links ]
4. United Nations. Declaration of Commitment on HIV/AIDS. UN General Assembly Special Session on HIV/AIDS. New York: United Nations, 2001. [ Links ]
5. Brocklehurst P, Volmink J. Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection. Cochrane Database of Systematic Reviews 2002; Art. No.: CD003510. [ Links ]
6. Health Systems Trust. Court ruling favours children orphaned by AIDS. Health Systems Trust News http://www.hst.org.za/news/20031210 (accessed 10 July 2007). [ Links ]
7. Dabis F, Leroy V, Bequet L, et al. Effectiveness of a short course of zidovudine + nevirapine to prevent mother-to-child transmission (PMTCT) of HIV-1: The Ditrame Plus ANRS 1201 Project in Abidjan, Cote d'Ivoire. XIV International AIDS Conference, Barcelona, 7-12 July 2002. [ Links ]
8. Department of Health of the Western Cape. Prevention of Mother to Child Transmission Protocol. Cape Town: Provincial Department of Health, 2004. [ Links ]
9. Delva W, Draper B, Temmerman M. Implementation of single-dose nevirapine for prevention of MTCT of HIV - lessons from Cape Town. S Afr Med J 2006; 96: 706-709. [ Links ]
10. Department of Health. National HIV and Syphilis Prevalence Surveys 2003 & 2004. Pretoria: Department of Health, 2004. [ Links ]
11. Human I, Kroon M, Bergman N, Fawcus S. Patient population movement in a Cape Town obstetric service. S Afr Med J 2003; 93: 634. [ Links ]
12. Ekouevi DK, Tonwe-Gold B, Dabis F. Advances in the prevention of mother-to-child transmission of HIV-1 infection in resource-limited settings. AIDS Read 2005; 15: 479-480, 487-493. [ Links ]
13. Sherman G, Jones SA, Coovadia AH, Urban M, Bolton K. PMTCT programme - partial assessments can build the picture. S Afr Med J 2004; 94: 934. [ Links ]
14. Violari A, Fiamma A, Duvenhage M, Gray GE, McIntyre J. One year out: experiences in a PMTCT program in an urban African setting. 3rd International AIDS Society Conference, Rio de Janeiro, Brazil, 24-27 July 2005. [ Links ]
15. McIntyre J. Preventing mother-to-child transmission of HIV: successes and challenges. BJOG 2005; 112: 1196. [ Links ]
16. World Health Organization. Antiretroviral Drugs for Treating Pregnant Women and Preventing HIV Infection in Infants: Towards Universal Access. Geneva: World Health Organization, 2006. [ Links ]
 Correspondence:
Correspondence:
B Draper
(bevvdraper@iafrica.com)
Accepted 21 April 2008.














