Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.98 no.4 Pretoria Abr. 2008
SAMJ FORUM
CLINICAL PRACTICE
Co-trimoxazole prophylaxis in HIV: The evidence
T YoungI; C E M OliphantIII; I AraoyinboI; J VolminkI, II
IDrs Young and Araoyinbo and Professor Volmink are staff members of the South African Cochrane Centre (an intramural unit of the South African Medical Research Council) which is part of the international Cochrane Collaboration, a non-profit organisation operating worldwide, which disseminates up-to-date reviews on the effects of health care interventions in order to help people make well-informed decisions
IIProfessor Volmink is also the Deputy Dean (Research) at the Faculty of Health Sciences, Stellenbosch University
IIIDr Oliphant is a Senior Medical Superintendent at Tygerberg Hospital
Human immunodeficiency virus (HIV) damages the body's immune system, making secondary (or opportunistic) infections more common. Treatment and prevention of such infections is integral to the management of patients with HIV infection. Co-trimoxazole is a prophylactic treatment that has a wide range of action against common bacteria, parasites, fungi and yeasts. As part of a minimum care package, UNAIDS/ WHO recommends co-trimoxazole prophylaxis for HIV-infected adults with symptomatic disease (WHO stage II, III or IV), or asymptomatic individuals with CD4 counts <500 cells/µl, and for all HIV-positive pregnant women after the first trimester.1 Co-trimoxazole is also recommended for use in children with proven HIV infection and infants exposed to HIV (from 4 - 6 weeks of age until infection with HIV is ruled out).2
The object of this report is to summarise the effects of co-trimoxazole prophylaxis on morbidity and mortality among HIV-infected individuals.
Beneficial effects
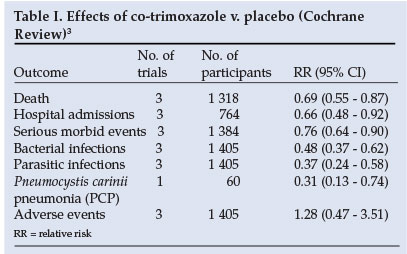
In HIV-positive adults not receiving antiretroviral therapy (ARV), a Cochrane Review (including three randomised controlled trials (RCTs)) found that co-trimoxazole prophylaxis reduced the risk of death by almost a third (Table I).3 The beneficial effect was similar for early (CD4 >200 cells/µl) and advanced (CD4 <200 cells/µl) disease. The frequency of admissions to hospital and the incidence of bacterial, parasitic and Pneumocystis carinii pneumonia (PCP) infections were also significantly reduced (Table I). A further RCT among HIV-positive adults in Malawi newly diagnosed with tuberculosis4 found no significant difference in bacterial pneumonia (hazard ratio (HR) 1.07 (95% confidence interval (CI) 0.56 - 2.06)) and the probability of survival (HR 1.11 (95% CI 0.72 - 1.71)) between participants allocated 480 mg v. 960 mg of co-trimoxazole.
In children, support for co-trimoxazole prophylaxis came from a randomised placebo-controlled trial (N=541) conducted in an area in Zambia with high levels (60 - 80%) of in vitro resistance to this antibiotic.5,6 Children <5 years were given a daily dose of 240 mg co-trimoxazole while those >5 years received a daily dose of 480 mg. Co-trimoxazole significantly reduced mortality by 33% (RR 0.67; 95% CI 0.53 - 0.85) and hospital admission rates by 23% (RR 0.77; 95% CI 0.62 - 0.95). Follow-up was reported to be excellent and few patients stopped their medication. The beneficial effect was seen across all ages and CD4 counts, and effectiveness of the drug did not diminish during periods of use up to 18 months' administration.
Harmful effects
The Cochrane Review found a higher risk of adverse effects in adults on co-trimoxazole compared with placebo, but this difference was not statistically significant (Table I). The RCT in Zambian children found no difference between treatment and control groups in the incidence of one or more grade 3 or 4 adverse drug reactions (HR 0.76; 95% CI 0.39 - 1.5). No allergic reactions to co-trimoxazole occurred in this study. In HIV-infected patients with a previous history of mild or moderate hypersensitivity to co-trimoxazole who required prophylaxis, desensitisation (stopping treatment and recommencing treatment with dose escalation over a period of days) compared with co-trimoxazole rechallenge (stopping treatment and starting at the full dose) resulted in fewer treatment discontinuations before 6 months (RR 0.64; 95% CI 0.45 - 0.91) and overall adverse reactions (RR 0.51; 95% CI 0.36 - 0.73).7
Comments
No randomised studies provide information on the optimal time for initiating prophylaxis in adults, or on when to stop prophylaxis. None of the trials included in the review focused on patients receiving treatment with antiretrovirals. Current studies neither report on the effects of prolonged co-trimoxazole use on bacterial resistance nor evaluate whether co-trimoxazole affects resistance of malaria parasites to sulfadoxine pyrimethamine (with which co-trimoxazole shares a component).
Conclusions
Co-trimoxazole is highly effective in reducing mortality and morbidity in HIV-infected adults and children not receiving antiretroviral treatment. Similar benefits are seen in early and advanced HIV disease. Co-trimoxazole is well tolerated, with minimal side-effects. Further research is required on the optimal time for commencement of co-trimoxazole prophylaxis and to evaluate its use in patients on antiretrovirals.
We thank F Desai, E Goemaere, Gail Kennedy and George Rutherford for their valuable feedback.
1, Provisional WHO/UNAIDS secretariat recommendations on the use of cotrimoxazole prophylaxis in adults and children living with HIV/AIDS in Africa. Report 29/03/2000. Geneva: World Health Organization, 2000. [ Links ]
2. Joint WHO/UNAIDS/UNICEF statement on use of cotrimoxazole as prophylaxis in HIV exposed and HIV infected children. Press statement 22 November 2004. Geneva: World Health Organization, 2004. [ Links ]
3. Grimwade K, Swingler, G. Cotrimoxazole prophylaxis for opportunistic infections in adults with HIV. The Cochrane Database of Systematic Reviews 2003, Issue 3. Art. No.: CD003108. DOI: 10.1002/14651858.CD003108. [ Links ]
4. Boeree MJ, Sauvageot D, Banda HT, Harries AD, Zijlstra EE. Efficacy and safety of two dosages of cotrimoxazole as preventive treatment for HIV-infected Malawian adults with new smear- positive tuberculosis. Trop Med Int Health 2005; 10(8): 723-733. [ Links ]
5. Chintu C, Bhat GJ, Walker AS, et al. Co-trimoxazole as prophylaxis against opportunistic infections in HIV-infected Zambian children (CHAP): a double-blind randomised placebo-controlled trial. Lancet 2004; 364: 1864-1871. [ Links ]
6. Grimwade K, Swingler GH. Cotrimoxazole prophylaxis for opportunistic infections in children with HIV infection. The Cochrane Database of Systematic Reviews 2006, Issue 1. Art. No.: CD003508. DOI: 10.1002/14651858.CD003508.pub2. [ Links ]
7. Lin D, Li WK, Rieder MJ. Cotrimoxazole for prophylaxis or treatment of opportunistic infections of HIV/AIDS in patients with previous history of hypersensitivity to cotrimoxazole. The Cochrane Database of Systematic Reviews 2007, Issue 2. Art. No.: CD005646. DOI:10.1002/14651858. CD005646.pub2. [ Links ]
 Correspondence:
Correspondence:
T Young
(taryn.young@mrc.ac.za)














