Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
SAMJ: South African Medical Journal
versão On-line ISSN 2078-5135
versão impressa ISSN 0256-9574
SAMJ, S. Afr. med. j. vol.98 no.3 Pretoria Mar. 2008
GUIDELINES
Influenza Guideline for South Africa Update 2008
R J Green; C Feldman; B Schoub; G A Richards; S A Madhi; H J Zar; U Lalloo
On behalf of the original Guideline Committee of MCMXCIX
ABSTRACT
OBJECTIVE: The South African Thoracic Society, in conjunction with interested stakeholders, published a Guideline for Influenza Management in Adults in 1999. This year the South African Thoracic Society (SATS) identified the need to revise that guideline for the following reasons:
- To indicate the viral strains that are to be incorporated into the vaccine for the 2008 season
- To add important new data regarding treatment of influenza
- To add a section on influenza in children
- To clarify issues in managing and preventing influenza in HIV-infected individuals.
INFLUENZA VIRUS: The influenza virus genus belongs to the family orthomyxoviridae. The haemagglutinin (HA) protein is the outermost protein, responsible for attachment to the host receptor, and is critical in determining the host's immune response to the virus. Changes in the antigenic epitopes of HA therefore allow the virus to escape the host's specific immune response. The genus is classified into three types, A, B and C, on the basis of the antigenic epitopes of the nucleoprotein (NP). Type A, which is widespread in nature in birds and mammals, is the most important type clinically and epidemiologically. It is further divided into subtypes on the basis of the antigenic epitopes of the HA and neuraminidase (NA) proteins. Each of the human subtypes H1N1, H2N2 and H3N2 are further subdivided into strains on the basis of more subtle antigenic properties of the HA protein.
INFLUENZA VACCINATION: Influenza vaccine is the mainstay of influenza prevention strategies. All persons who are at high risk of influenza and its complications because of underlying medical conditions or who are receiving regular medical care for conditions such as chronic pulmonary and cardiac disease, chronic renal diseases, neuromuscular diseases, diabetes mellitus and similar metabolic disorders, and individuals who are immunosuppressed (including HIV-infected persons with CD4 counts above 100 cells/µl and HIV-infected children with CD4 counts >15%), should be vaccinated. Vaccines should be given from at least 2 months prior to the onset of autumn (March in South Africa). The recommended vaccine formulation for 2008 is:
- A/Solomon Islands/3/2006 (H1N1) (IVR-145)
- A/Brisbane/10/2007 (H3N2) (IVR-147)
- B/Florida/4/2006 or B/Brisbane/3/2007.
TREATMENT OF INFLUENZA: Influenza illness is characterised by the acute onset of systemic and respiratory signs occurring in autumn or winter. Recommendations for the Prevention and Control of Influenza have indicated that neither amantadine nor rimantadine should be used for the treatment or chemoprophylaxis of influenza A. NA inhibitors are an important adjunct to influenza vaccination, in both the prevention and treatment of influenza. Because of concerns about the possibility of the development of viral resistance with overuse of these agents, it is recommended that NA inhibitors in the treatment of influenza should be reserved for high-risk or sicker influenza patients.
1. Objective
The South African Thoracic Society, in conjunction with interested stakeholders, published a Guideline for Influenza Management in Adults in 1999.1 This year the South African Thoracic Society (SATS) identified the need to revise that guideline for the following reasons:
- To indicate the viral strains that are to be incorporated into the vaccine for the 2008 season
- To add important new data regarding treatment of influenza
- To add a section on influenza in children
- To clarify issues in managing and preventing influenza in HIV-infected individuals.
2. Influenza the virus
2.1 Structure
Influenza virus is a middle-sized virus about 80 - 120 nm in diameter. Like all viruses it consists of a genome covered by protein shell (capsid) and, being an enveloped virus, by a surrounding bilipid layer derived from the cellular cytoplasmic membrane as the virus buds out of the host cell at the end of its replication cycle. Because of the loose envelope, the shape of the virus is pleomorphic, varying from spherical to tubular.
The genome consists of a single-stranded RNA of negative polarity (i.e. the viral RNA or vRNA is not translated directly into protein but via an intermediate mirror-image complementary RNA or cRNA strand), so that vRNA transcribes to cRNA which then translates to protein. Replication of the RNA is mediated by a viral enzyme, RNA-dependent RNA polymerase, also known as RNA replicase.
Covering the genome, and closely attached to it, is the nucleoprotein (NP) which is in turn covered by the matrix protein (M), the major protein bulk of the virus. The matrix gene codes for two matrix proteins, M1, which is the major component of the capsid, and M2, a protein embedded in the envelope of the virus, which forms an ion exchange channel that facilitates the penetration of the virus into the host cell. In addition there are two non-structural viral proteins, NS1 and NS2.
A lipid bilayer is acquired by the virus from the host cell cytoplasmic membrane as it buds out of the host cell to infect new cells after its replication. Embedded in this envelope are the two viral spikes, also referred to as peplomers, haemagglutinin (H or HA) and neuraminidase (N or NA).
HA is a rod-shaped trimeric protein. Each monomer comprises two segments, a proximal HA2 component which is embedded in the viral envelope, and a distal HA1 component which attaches to (sialic) neuraminic acid receptor of the host. The cleavage site between HA1 and HA2 consists of a short oligopeptide sequence called the fusion peptide. In the highly pathogenic forms of avian influenza (HPAI) this cleavage site consists of basic amino acids; this is used as a diagnostic marker of HPAI. The HA protein also attaches to red blood cells causing them to clump together, i.e. haemagglutination; this forms the basis of a commonly used serological test for the virus, the haemagglutination test, or for antibodies to the virus, the haemagglutination inhibition test.
The other surface spike protein, NA, is a mushroom-shaped protein with its stalk embedded in the virus envelope. Being an enzyme, NA's function is to digest the cell surface neuraminic acid to free the virus from the cell after its replication cycle and allow it to infect further cells.
The HA protein, being the outermost protein and responsible for attachment to the host receptor, is critical in determining the host's immune response to the virus. Changes in antigenic epitopes of HA allow new viral infections to escape preexisting humoral responses induced by non-matching strains of influenza virus. To a lesser extent, the immune response to NA is also responsible for protection and also for recovery from infection.
2.2 Classification
The influenza virus genus belongs to the family orthomyxoviridae. The genus is classified into three types, A, B and C, on the basis of the antigenic epitopes of the NP protein. Type A, which is widespread in nature in birds and mammals, is the most important type clinically and epidemiologically. It is further divided into subtypes on the basis of the antigen sequence diversity of the HA and NA proteins. In nature some 16 HAs and 9 NAs have been described, all of which are found in birds, while relatively few have been detected in mammals. In man only H1, H2 and H3 and N1 and N2 in the combinations H1N1, H2N2 and H3N2 have so far been associated with regular outbreaks of human influenza. Only on rare occasions have the non-human viruses crossed the species barrier from birds to infect humans, but these have only caused sporadic cases of influenza and have not established themselves in the human host to the extent of being transmissible between humans.
Types B and C influenza virus are found exclusively in humans. They are not classified into subtypes, but each type is subdivided into strains. Type C is mainly a cause of minor upper respiratory tract infection and is therefore not included in the vaccine.
The nomenclature for influenza strains details, in sequence, the type, subtype (in the case of type A) and the strain identifiers - the place where it was first characterised, the year of isolation and a laboratory identifying number. The following strains that have been identified by the World Health Organization for the 2008 Southern Hemisphere influenza season are set out in Table I.2

3. Influenza vaccine strategy
Influenza vaccine forms the most important basis for prevention of influenza disease. Vaccines should contain 15 µg of each HA antigen in each 0.5 ml dose. Tables II and III list the indications for influenza vaccine in adults and children, respectively. Table IV lists the influenza vaccine dosages and Table V the contraindications to vaccination. Vaccination should be given sufficiently early to provide protection for the autumn and winter. A protective antibody response takes about 2 weeks to develop. However, vaccination can and should be done at any time of the season, even until late winter.




4. Clinical disease in adults
Influenza occurs in autumn and winter in South Africa. Cases are reported from April to October. A clinical diagnosis of influenza is usually sufficient. In adults sudden onset of fever and cough during the influenza season has been reported to have a positive predictive value of 79%.3 The main underlying disorders associated with increased risk of complications from influenza are chronic respiratory and cardiac conditions (Table VI).

5. Clinical disease in children
Influenza illness is characterised by the acute onset of systemic and respiratory signs.3,4 The abrupt onset and systemic signs in a child during the influenza season should enable influenza to be distinguished from a simple upper respiratory tract infection or common cold. Common symptoms and signs of influenza infection are listed in Table VII.

In infants and neonates nonspecific signs or fever alone may be the only presenting feature. For most children under 2 years of age, a clinical case definition includes sudden onset of high fever, cough and rhinorrhoea during the influenza season.5
For older children (>2 years) sudden onset of fever, cough, pharyngitis and headache during the influenza season have been reported to have a sensitivity and specificity of approximately 80% each.6 Other symptoms or signs include fatigue (51 - 75%), chills (76 - 100%), myalgia or conjunctivitis (26 - 50%).
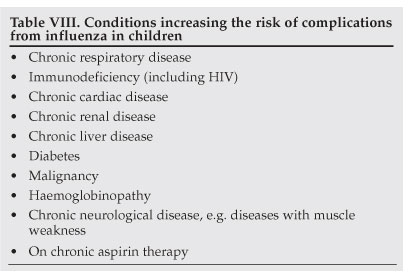
The risk of complications from influenza is higher in young children than in adults, with approximately 20 - 30% of children under 2 years of age developing complications.3,4 In addition, children with underlying chronic illnesses are at increased risk of complications (Table VIII).
Common complications include febrile convulsions, otitis media, sinusitis, bronchiolitis, croup and pneumonia.4 Pneumonia may be due to viral infection or secondary bacterial infection, especially with Streptococcus pneumoniae. The rates of hospitalisation and death are increased in children under 2 years of age and especially in those under 6 months of age. Reye's syndrome, encephalitis, pericarditis and myocarditis are rarely reported complications.4 Influenza virus infection may exacerbate an underlying chronic illness.
Influenza is a common (5 - 10%) cause of pneumonia and bronchiolitis in children and may be indistinguishable from other viral infections (such as respiratory syncytial virus (RSV)).6,7 Distinguishing influenza from illness caused by other respiratory viruses may be difficult on the basis of signs and symptoms, and laboratory diagnostic confirmation may be useful.
6. Antiviral agents for influenza
The antiviral agents currently available for use include the NA inhibitors and M1 inhibitors (adamantanes).
6.1 M1 inhibitors (adamantanes)
Recent evidence has indicated that a high proportion of the circulating influenza A strains in the USA are resistant to amantadine (as well as to rimantadine, which is not available in South Africa). For this reason more recent editions of the Advisory Committee on Immunization Practices (ACIP) Recommendations for the Prevention and Control of Influenza have indicated that neither amantadine nor rimantadine should be used for the treatment or chemoprophylaxis of influenza A in the USA.3 Currently, therefore, only NA inhibitors are recommended for antiviral treatment or chemoprophylaxis of influenza in the USA.
6.2 NA inhibitors
6.2.1 Treatment of influenza
NA inhibitors are an important adjunct to influenza vaccination, in both the prevention and treatment of influenza. They act by specifically inhibiting the NA enzymes that are present on all influenza subtypes and are responsible for releasing viral particles from host cells and the spread of infection. Two NA inhibitors are currently available for clinical use, oseltamivir (Tamiflu) and zanamivir (Relenza). Oseltamivir is given orally as a prodrug and is distributed systemically to all potential infection sites. Zanamivir is administered via inhalation and is deposited primarily in the respiratory tract.
When given within 48 hours of onset of symptoms of influenza, both agents significantly reduce duration of illness and symptom severity, and decrease the rate of influenza-associated complications, such as pneumonia, bronchitis and otitis media.3 The current approved indications for oseltamivir are for the treatment of uncomplicated acute illness due to influenza infection in patients ≥1 year of age or older, who have been symptomatic for 48 hours or less (but British Thoracic Society (BTS) guidelines also recommend that oseltamivir can be used up to 5 days into hospitalisation). The recommended dose in adults is 1 capsule (75 mg) twice daily for 5 days for treatment of influenza and 1 capsule per day to a maximum of 6 weeks for prophylaxis. Oseltamivir is well tolerated and the most common side-effect has been mostly mild nausea or vomiting. Other side-effects are rare and include headache, fatigue, insomnia and dizziness.
Current data indicate that oseltamivir significantly reduces the complication rate from influenza in children by up to 40%, and reduces the need for hospitalisation, the duration of hospitalisation in children already hospitalised and the duration of illness.8-11 Early diagnosis is essential as treatment with oseltamivir should be initiated within the first 48 hours of the onset of illness.4,9,10 Oseltamivir is indicated for treatment of influenza in children older than 1 year of age to reduce the risk of complications and severity of illness. Oseltamivir is specifically indicated in children with conditions listed in Table IX.10,11

Dosage of oseltamivir in children
Oseltamivir is available as a capsule (75 mg) or syrup (12 mg/ml). The recommended dosage is shown in Table X.

Zanamivir is similarly approved for the treatment of uncomplicated illness due to influenza infection in patients ≥12 years of age or older, who have been symptomatic for 48 hours or less. The recommended dose of zanamivir for the treatment of influenza is two inhalations (one 5 mg blister per inhalation for a total dose of 10 mg) twice daily. Zanamivir is generally well tolerated, although there have been episodes of decrease in lung function and/or bronchospasm in patients with asthma and/or underlying airway diseases. Care is needed when using zanamivir in patients with underlying airway diseases. Individuals with asthma or chronic obstructive pulmonary disease (COPD) who are using zanamivir should always have a short-acting bronchodilator available when inhaling zanamivir and should discontinue therapy and contact their physician if they experience difficulty in breathing.
It has also been recommended that both oseltamivir and zanamivir be made part of pandemic influenza preparedness, such as for the H5N1 influenza virus ('bird 'flu'). There is evidence of ongoing susceptibility of this influenza strain to the NA inhibitors. While both may potentially be of benefit, it has been suggested that oseltamivir may be particularly useful because of its systemic absorption, and the systemic nature of H5N1 infection.
Because of concerns about the possibility of resistance developing to these agents with overuse, it is recommended that their use be reserved for sicker influenza patients or for prophylaxis in high-risk patients. They must not replace vaccination for prophylaxis (except in the very rare cases where vaccine is contraindicated in high-risk patients), but could supplement vaccination in high-risk individuals.
Additional treatment for influenza in children
Supportive and symptomatic therapy should be given as clinically indicated.4 Oxygen is a mainstay of treatment in hypoxic patients. Children or adults with suspected secondary bacterial infection or who are severely ill with pneumonia requiring hospitalisation should also be treated with an antibiotic for community-acquired pneumonia according to national guidelines.4,6
6.2.2 Prophylaxis of influenza
Oseltamivir is also effective in adults and adolescents (>13 years old), protecting close contacts of index cases from symptomatic influenza when used as post-exposure prophylaxis. Prophylaxis can be post-exposure (prophylaxis for ≥7 days after exposure to infected individuals), seasonal (for the entire influenza season, e.g. in individuals in whom vaccination is contraindicated), post-vaccination (prophylaxis for 2 - 4 weeks after receiving vaccination) and for outbreak control (e.g. institutional outbreak). Prophylaxis is recommended for high-risk individuals.
7. Diagnostic testing
While most cases of influenza are diagnosed clinically, rapid tests (Immunocard, Binax Now Influenza A&B, Directigen EZ Flu A+B, Denka Seiken Quick Ex-Flu, Fujirebio Espline Influenza A&B-N, and Quidel QuickVue Influenza A+B Test) are available in South Africa. The sensitivity and specificity of locally available testing should be discussed with local laboratory experts when requesting these tests. In general, rapid tests have a sensitivity of approximately 70%; a negative test should therefore be followed by culture. Conventional testing of respiratory secretions by immunofluorescence or realtime polymerase chain reaction (RT-PCR) is offered by most laboratories. Cell culture is used for epidemiological purposes. The sensitivity of diagnostic assays, especially culture and immunofluorescence, differs between age groups and they are more sensitive among children, in whom shedding of influenza virus is more prolonged (7 - 14 days) than in adults. A negative culture/immunofluorescence test does not rule out the diagnosis of influenza infection in adults, among whom paired serology assays may be more sensitive, but clearly not useful in selecting therapy.
8. Influenza and asthma
Influenza virus continues to be a major cause of respiratory infection and is an important contributor to morbidity and mortality in populations at risk, including those with underlying pulmonary conditions such as asthma. Vaccination with inactivated influenza vaccine remains the most popular method of controlling influenza through prevention. Patients with moderate to severe asthma should be advised to receive an influenza vaccination every year, or at least when vaccination of the general population is advised. However, routine influenza vaccination of children and adults with asthma does not appear to protect them from asthma exacerbations or improve asthma control.12,13
9. Influenza and HIV
Influenza tends to produce more severe disease in HIV-infected individuals. The incidence of severe pneumonia in which influenza virus was identified was 8.03-fold (95% confidence interval (CI) 5.05 - 12.76) greater in HIV-infected than in HIV-uninfected children aged <2 years.14 Additionally, whereas RSV was the most commonly identified virus (18.1%) in HIV-uninfected children hospitalised for pneumonia, the frequency of identification of influenza virus (5.4%) was the same as that for RSV (5.3%) in HIV-infected children hospitalised for pneumonia.15 Similar to high-risk HIV-uninfected individuals, the mainstay for the prevention of influenza virus infections in HIV-infected individuals is immunisation with the trivalent sub-unit vaccines. Concerns regarding the use of the sub-unit vaccines, which elicit a T-cell-dependent immune response, centred around the transient increase in HIV viral load and decreases in CD4+ lymphocyte counts observed after vaccination in HIV-infected individuals, are considered not to be clinically significant in adults or children.14-16
In summary, vaccination of HIV-infected individuals with the sub-unit influenza vaccine is safe; however, it induces only moderate immune responses and is modest in the protection it confers compared with HIV-uninfected individuals. On the basis of the available data, influenza vaccination should be considered for adult HIV-infected individuals and has been found to be particularly effective in those with CD4 counts >100 cells/µl. For children evidence of efficacy is lacking. While some guidelines7 suggest that all HIV-infected children should receive influenza vaccine, it seems likely that the best response will be in those with a CD4 count of >15%. Similarly, pneumococcal conjugate vaccine in HIV-infected children should be encouraged to prevent superimposed bacterial complications of influenza virus infections.
10. Avian influenza
Pandemics caused by highly pathogenic, avian-derived influenza A viruses have been documented since the 12th century.17,18 The most recent of these was the global disaster of 1918 - 1919 in which up to 50 - 100 million people died.19 These pandemics are related to antigenic shift. The prerequisites for the development of a pandemic are that there should be a novel virus, that it should be able to cross the species barrier causing virulent infection, and finally that there should be efficient human-to-human transmission. Fortunately most avian strains, even novel viruses such as the H7N7 strain that recently emerged in Holland, have relatively low pathogenicity.20
However, in 1997 in Hong Kong, H5N1, a highly pathogenic variety, was identified as a human infection transmitted from birds, and at this time a pandemic was probably averted or significantly delayed by the destruction of the entire domestic bird population in Hong Kong.21 In 2003 antigenic drift occurred causing pathogenicity to a larger number of species, conferring resistance to amantadine and rimantadine, and greatly increasing the virulence. The initial outbreak of human disease in 2003 occurred in Vietnam and remained confined to south-east Asia until 2005, when the virus was spread by wild birds to domestic bird populations in Europe, Africa and the Middle East.22 This has significantly increased the numbers of people exposed to the virus and commensurately increased the potential for mutation to a form that is transmitted between humans in an efficient and sustained manner. Cases of human-to-human transmission have however remained sporadic and inefficient.23 As of 5 November 2007, there have been 334 cases of H5N1 influenza with 205 deaths in 12 countries (including Djibouti, Egypt and Nigeria), a mortality rate of 60%.24
Pandemic influenza differs from that of the seasonal variety in which death occurs predominantly in the elderly or those with co-morbid disease. In 1918 the age-specific mortality was highest for those in their teens to 3rd decade.25 In the 205 human cases that occurred between December 2003 and April 2006 the mean age was 20 years (range 3 months - 75 years); 50% were aged under 20 and 90% under 40.26 The overall case fatality rate was 56%, with the highest mortality (73%) in the 10 -19-year age group (N=49) and the lowest (18%) in those aged over 50 (N=11).26
Calculation of excess mortality in the 1918 - 1920 pandemic from countries that had high-quality registration data shows that mortality varied more than 30-fold across countries and that per-head income explained most of the variation. Extrapolation of these data to 2004 indicates that a similar pandemic would kill ±62 million (51 million - 81 million), and that 96% (95% CI 95 - 98) of deaths would occur in the developing world. If these deaths occurred in a single year, they would increase global mortality by 114%.27
In the event of a pandemic, control by means of case tracing and isolation will be impossible as virus is shed prior to the development of symptoms.28 Infection control specialists and virologists will be valuable primarily to inform clinicians of the arrival of H5N1 and that patients should thereafter be treated presumptively. The WHO has developed a national preparedness plan and has recommended that these be rapidly developed, practised and adapted to each specific country.29 The most important intervention, however, would be an effective, widely available and cheaply produced vaccine, unfortunately an unlikely scenario in the near future. Antivirals would represent the second most important intervention. Mathematical modelling of a pandemic similar to 1918, in which the mortality was estimated to be 0.5% across all three waves, indicated that a stockpile capable of treating 20% of the population would result in a mortality reduction of 53%.30 Excluding the issue of efficacy, the primary problems with the use of these NA inhibitors will be early diagnosis of the disease, as currently available PCR testing though rapid will probably be rendered impractical in the face of massive morbidity,31 the logistics of distribution within the 36-hour window period, and the potential for the virus to develop resistance.
All health care workers must be educated with regard to the risks of droplet spread of infection to staff and other patients, and trained in infection control and disease management. It is critically important that all health care workers and others involved in essential services are confident that everything possible has been done to protect them and their families, as fear and demoralisation will compromise their efficiency and increase absenteeism. Preparations should be made to minimise human-to-human contact, for school, company, theatre and cinema closures, and for restriction of mobility within the community. Masks must be sourced and plans made for distribution. Large employers should develop response plans similar to the national recommendations of the WHO.29
Avian influenza has the potential to cause the biggest health care disaster since the 1918 influenza pandemic. Preparation should begin now to ameliorate the effects as much as possible.
11. References
1. Feldman C, Klugman K, Phillips D, et al. Adult Influenza Vaccination Guideline. S Afr Med J 1999; 89: 1216-1222. [ Links ]
2. Department of Health. Recommendations pertaining to the use of viral vaccines: Influenza 2008 (Drug Alert). S Afr Med J 2008; 98: 84. [ Links ]
3. Monto AS, Gravenstein S, Elliott M, Colopy M, Schweinke J. Clinical signs and symptoms predicting influenza infection. Arch Intern Med 2000; 160: 3043-3247. [ Links ]
4. Fiore AE, Shay DK, Haber P, Iskander JK, Uyeki TM, Mootrey G, Bresee JS, Cox NJ; Advisory Committee on Immunization Practices (ACIP), Centers for Disease Control and Prevention (CDC). Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR Recomm Rep 2007; 56(RR-6): 1-54. [ Links ]
5. British Infection Society; British Thoracic Society; Health Protection Agency. Pandemic flu: clinical management of patients with an influenza-like illness during an influenza pandemic. Provisional guidelines from the British Infection Society, British Thoracic Society, and Health Protection Agency in collaboration with the Department of Health. Thorax 2007; 62: Suppl 1, 1-46. [ Links ]
6. Friedman MJ, Attia MW. Clinical predictors of influenza in children. Arch Pediatr Adolesc Med 2004; 158: 391-394. [ Links ]
7. Zar HJ, Jeena P, Argent A, Gie R, Madhi S. Diagnosis and management of community acquired pneumonia in childhood - South African Thoracic Society Guidelines. S Afr Med J 2005; 95: 977-990. [ Links ]
8. Whitley RJ, Hayden FG, Reisinger KS, et al. Oral oseltamivir treatment of influenza in children. Pediatr Infect Dis J 2001; 20(2): 127-133. [ Links ]
9. Barr CE, Schulman K, Iacuzio D, Bradley JS. Effect of oseltamivir on the risk of pneumonia and use of health care services in children with clinically diagnosed influenza. Curr Med Res Opin 2007; 23(3): 523-531. [ Links ]
10. Cooper NJ, Sutton AJ, Abrams KR, Wailoo A, Turner D, Nicholson KG. Effectiveness of neuraminidase inhibitors in treatment and prevention of influenza A and B: systematic review and meta-analyses of randomised controlled trials. BMJ 2003; 326: 1235. [ Links ]
11. Townsend KA, Eiland LS. Combating influenza with antiviral therapy in the pediatric population. Pharmacotherapy 2006; 26(1): 95-103. [ Links ]
12. Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. Workshop Report. 2006; MCR Vision (publ). http://www.ginasthma.org (accessed 23 January 2008). [ Links ]
13. Hanania NA, Atmar RL, Castro M. Influenza vaccine in patients with asthma. Expert Rev Vaccines 2006; 5(1): 111-118. [ Links ]
14. Glesby MJ, Hoover DR, Farzadegan H, Margolick JB, Saah AJ. The effect of influenza vaccination on human immunodeficiency virus type 1 load: a randomized, double-blind, placebo-controlled study. J Infect Dis 1996; 174(6): 1332-1336. [ Links ]
15. Madhi SA, Schoub B, Simmank K, Blackburn N, Klugman KP. Increased burden of respiratory viral associated severe lower respiratory tract infections in children infected with human immunodeficiency virus type-1. J Pediatr 2000; 137(1): 78-84. [ Links ]
16. Keller M, Deveikis A, Cutillar-Garcia M, et al. Pneumococcal and influenza immunization and human immunodeficiency virus load in children. Pediatr Infect Dis J 2000; 19(7): 613-618. [ Links ]
17. Cunha BA. Influenza: Historical aspects of epidemics and pandemics. Infect Dis Clin North Am 2004; 18(1): 141-155. [ Links ]
18. Wong SS, Yuen KY. Avian influenza virus infections in humans. Chest 2006; 129(1): 156-168. [ Links ]
19. Fedson DS. Preparing for pandemic vaccination: An international policy agenda for vaccine development. J Public Health Policy 2005; 26: 4-29. [ Links ]
20. WHO Epidemic and Pandemic Alert and Response (EPR). http://www.who.int/csr/disease/avian_influenza/en/ (accessed 22 April 2007). [ Links ]
21. Yuen K, Chan P, Peiris M, et al. Clinical features and rapid diagnosis of human disease associated with avian influenza A H5 N1 virus. Lancet 1998; 351: 467-471. [ Links ]
22. Avian Influenza fact sheet (WHO). http://www.who.int/mediacentre/factsheets/avian_influenza/en/index.html (accessed 25 July 2006). [ Links ]
23. Ungchusak K, Auewarakul P, Dowell SF, et al. Probable person-to-person transmission of avian influenza A (H5N1). N Engl J Med 2005; 352: 333-340. [ Links ]
24. Avian Influenza fact sheet (WHO). http://www.who.int/csr/disease/avian_influenza/country/cases_table_2006 _07_26/en/index.html (accessed 3 August 2006). [ Links ]
25. Nguyen-Van-Tam JS, Hampson AW The epidemiology and clinical impact of pandemic influenza. Vaccine 2003; 21: 1762-1768. [ Links ]
26. The Writing Committee of the World Health Organization Consultation on Human Influenza A/H5N1 Avian influenza A (H5N1) infection in humans. N Engl J Med 2005; 353: 1374-1385. [ Links ]
27. Murray CJ. Towards good practice for health statistics: lessons from the Millennium Development Goal health indicators. Lancet 2007; 369: 862-873. [ Links ]
28. Monto AS. The role of antivirals in the control of influenza. Vaccine 2003; 21: 1796-1800. [ Links ]
29. Epidemic alert and response: WHO checklist for influenza pandemic preparedness planning. WHO Department of Communicable Disease Surveillance and Response Global Influenza programme. http://whqlibdoc.who.int/hq/2005/WHO_CDS_CSR_GIP_2005.4.pdf (accessed 1 February 2006). [ Links ]
30. Gani R, Hughes H, Fleming D, et al. Potential impact of antiviral drug use during influenza pandemic. Emerg Infect Dis 2005; 11: 1355-1362. [ Links ]
31. Le QM, Kiso M, Someya K, et al. Avian flu: isolation of drug-resistant H5N1 virus. Nature 2005; 437: 1108. [ Links ]
12. SAMA-SAPS Adult Respiratory Vaccinations Adult Working Group 199
Chairperson:
Prof. J Milne
Authors:
Prof. C Feldman
Prof. K Klugman - Department of Health, Pharmacy Directorate (EDL)
Mrs D Phillips - Department of Health, Directorates, Communicable Diseases and Chronic Diseases, Disabilities and Geriatrics
Dr N A Cameron - Department of Health, National Advisory Group on Immunisation
Dr R R Eggers
National Institute for Virology
Dr D J Martin
Dr B Schoub
Infectious Diseases Society of SA
Dr A S Karstaedt
Dr S Waner
Prof. G Maartens
Prof. R Smego
National Pathology Group
Dr P Cole
Nominated Working Group Members
Prof. G Maartens
Dr R Wood
Prof. E D Bateman
Prof. R Smego
Dr D J MartinDr M Greenblatt
Prof. U G Lalloo
Dr S Waner - Pharmacy Association of SA
Mr J Bothma - South African Academy of Family Practice
Dr L Geffen - SAPS
Prof. C Feldman
Prof. U Lalloo - South African
Pulmonology Society
Dr R Green
South African Geriatric Society
Dr G Muller
South African Society of Occupational Medicine
Dr J Murphy
SAMA Guideline Committee Nominees
Mr T Groom
Dr A Ratsela
SAMA Centre of Quality Care Representative
Ms V Pinkney-Atkinson
SAMA General Practice Committee
Dr T Mbengashe
Sponsors
Pasteur Mérieux Connaught
(Rhône-Poulenc Rorer)
Ms E Robertson
Mr B Breenblatt
Mr M Ferraci
Ms C Lionnet
 Correspondence:
Correspondence:
Professor R J Green
Department of Paediatrics and Child Health, University of Pretoria
PO Box 667, Pretoria, 0001
tel. 012 3545272
e-mail: robin.green@up.ac.za














