Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Education
On-line version ISSN 2076-3433
Print version ISSN 0256-0100
S. Afr. j. educ. vol.39 n.3 Pretoria Aug. 2019
http://dx.doi.org/10.15700/saje.v39n3a1602
ARTICLES
Instructional curriculum based on cooperative learning related to the structure of matter and its properties: Learning achievement, motivation and attitude
Filiz Avcı; Fatma Gülay Kırbaşlar; Burçin Acar Şeşen
Faculty of Hasan Ali Yücel Education, İstanbul University-Cerrahpaşa, İstanbul, Turkey. filizfen@istanbul.edu.tr
ABSTRACT
In this study we aimed to analyse the effects of the Instructional Curriculum based on Cooperative Learning (ICBCL) prepared for the subjects, the orders of electrons and chemical properties, chemical bonds, compounds and their formulas, and mixtures on 7th grade learners' learning achievements, motivations to learn science, and their attitudes towards learning science. Pre- and post-test quasi experimental design was used in this study in which the participants, 89 7th grade learners were attending a public secondary school in Istanbul, Turkey during the 2013-2014 academic year. The Diagnostic Test for the Structure of Matter and its Properties (DTSMP), developed by the researchers, the Motivation Scale to Learning Science (MSTLS), developed by Dede and Yaman (2008), and the Attitude Scale Towards Science Lesson (ASTSL), developed by Biçer (2011) were used as data collection instruments. SPSS 16 and Lertap 5 were used for data analysis. As a result of the post-tests, learners from the experimental group achieved significantly higher mean scores than learners from the control group. This reflected that ICBCL was highly effective on increasing learners' achievement levels by preventing possible misconceptions, developing motivation, and positive attitudes compared to the current Science Teaching Curriculum (STC).
Keywords: attitudes towards science; cooperative learning; instructional curriculum; motivation to learn science; science education
Introduction
In the information age, education plays a major role in the economic development of any country. To ensure economic growth, especially in developing countries such as South Africa, Turkey, Cuba, and Algeria, instructional curriculums are being updated to address skills required of learners in the 21st century, namely knowledgeability about science, ability to use scientific processes, ability to communicate well with others, ability to dream big, being good at problem solving, caring about the environment, and respecting people's views (Partnership for 21st Century Skills, 2008).
The quality of education is closely related to the instructional design model used and how it is implemented when preparing the instructional curriculum (Erden, 1993). In most sources the concepts of Instructional Design and Instructional Systems Design are used interchangeably with regard to the instructional design model. Reigeluth (1999) describes these concepts as separate concepts. The concept of Instructional Design defines the whole of the strategies, methods, and techniques used during the design of a lesson, while the concept of Instructional Systems Design is the sum of activities of analysis, design, development, implementation, and evaluation during the design of a curriculum. Over the last decades, practitioners have developed a number of models about instructional system design (Lee, J & Jang, 2014).
The ADDIE model, a guide for creating an effective design (Aldoobie, 2015), is one of the most widely used models in teaching design. Educators need tools to teach knowledge, skills, and attitudes. Using the ADDIE model helps educators to perform these tasks (Cheung, 2016). When the steps of the ADDIE model are followed, it can be applied easily in online or face-to-face environments (Aldoobie, 2015).
Steps of the model are analysis, design, development, implementation and evaluation. In the analysis, learners' learning needs, limitations and present knowledge and skills are determined. In the design phase, the most appropriate environment is selected for the acquisition of knowledge and skills, and the teaching method, learning activities, and evaluation process are designed. In the development phase, teaching materials (all the tools to be used in teaching and support materials) are prepared and an appropriate learning environment is created. The product is developed at this stage. During application the design is fully implemented with real learners. During the evaluation the design is checked to determine how well the learning needs have been met by the learning objectives (Kaminski, 2007). Evidence of many studies using the ADDIE model for instructional design can be found in the literature (Arkün, 2007; Özerbaş & Kaya, 2017; Peterson, 2003; Reinbold, 2013). In this study, the relevant learning environment was developed based on the ADDIE instructional design model.
New instructional methods, techniques, and strategies, which will encourage individuals to think, discuss, research, question, think critically, and participate actively in the learning process, should be used in classroom settings when applying new instructional curriculums. One of the instructional methods in which learners actively participated, is cooperative learning. Cooperative learning is a learning method based on the cognitive developmental theory, the behavioral learning theory, the social interdependence theory, and the cognitive elaboration theory (Jacobs, 1990; Johnson & Johnson, 1999; Kauchak & Eggen, 2003; Slavin, 1995). Cooperative learning involves learners working on learning activities in small groups, getting intrinsic or instrumental awards as a result of the group's success and aims (Siegel, 2005). For cooperative learning to be successful it depends on positive cooperation, dependency, personal responsibilities, face-to-face communication, social skills, and the evaluation of cooperative work (Johnson & Johnson, 1999). When learners work cooperatively, they show increased participation at group argument, connect in fewer interruptions when others speak, and provide more intellectually valuable contributions (Gillies, 2006). Studies have shown that cooperative learning enables the development of social skills (Genlott & Grönlund, 2013), motivation for the lessons (Saban, 2004), positive attitudes towards autonomous learning (Johnson, Johnson & Smith, 2007), developing teamwork skills (Johnson & Johnson, 1999), development of face-to-face information sharing, helping, ,discussing and sharing skills (Tran & Lewis, 2012; Veenman, Van Benthum, Bootsma, Van Dieren & Van der Kemp, 2002).
Cooperative learning techniques have been widely used in science education around the World. Aruna and Sumi (2010), Ebrahim (2012), Fernandez-Rio, Sanz, Fernandez-Cando and Santos (2017); Lazarowitz (1991), Liao (2006), Lowe (2004), Marzban and Akbernejad (2013), Nam and Zellner (2011), Sisovic and Bojovic (2000), Tran and Lewis (2012), and Zoghi (2013) found that the cooperative learning method influenced achievement, attitudes, motivation, and scientific process skills, particularly in countries such as South Korea, New Zeland, Yugoslavia, Spain, Israeli, Kuwait, Vietnam, Iran and Taiwan. Cooperative learning is preferred to traditional learning methods due to greater learner achievement and development of social skills and less material needs (Carpenter & McMillan, 2003; Tarhan & Sesen, 2012).
One of the main subjects of science education is the learning of concepts. For this reason, the main aim of science education is to develop learners' understandings of scientific concepts. Effective science teaching requires constructing the concepts which are the building blocks of knowledge in learners' minds (Tatar & Cansüngü Koray, 2005). For reasons like biased thoughts, non-scientific beliefs, conceptual misunderstanding stemming from learners' background knowledge, misconceptions resulting from different uses in science and in daily life, and course book-related misconceptions, may prevent learners from constructing new knowledge (Köseoğlu, Atasoy, Kavak, Akkuş, Budak, Tümay, Kadayıfçı & Taşdelen, 2003). If new knowledge is not constructed well, it affects learning negatively and causes learner misconceptions (Jonassen, 1991).
It is well known that it is difficult for learners to understand science and chemistry as both contain a good number of abstract concepts (Gilbert, Justi, Van Driel, De Jong & Treagust, 2004; Yang, Andre, Greenbowe & Tibell, 2003). For example, macroscopic, microscopic, and symbolic are three levels used to express events in chemistry (Johnstone, 1993). The symbolic level refers to symbols, numbers, and formulae, while the micro dimension cannot be seen with the naked eye and the macro dimension covers events that learners can observe (Okumuş, Çavdar, Alyar & Doymuş, 2017). Learners know the three levels of chemistry but cannot make connections between three levels (Merritt, Shwartz & Krajcik, 2007). Since learners do not sufficiently relate to the symbolic, macro, and micro dimensions, they find it difficult to understand the structure of matter and its properties. Numerous studies show that learners at different levels (primary and secondary school) struggle to understand the structure of matter (Harrison & Treagust, 2003; Merritt et al., 2007). Structure of matter and its properties is very important in many of the subjects underlying chemistry (The Orders of Electrons and Chemical Properties, Chemical Bonds, Compounds and Their Formulas, Mixtures). In middle school, learners encounter these issues that form the basis of chemistry for the first time. In order to achieve success and to ensure success in the progressive education processes, these issues should be learned completely and without misunderstanding. Studies show that learners have a lot of misconceptions about the orders of electrons and chemical properties (Griffiths & Preston, 1992; Harrison & Treagust, 2000; Kara & Ergül, 2012), chemical bonds (Butts & Smith, 1987; Coll & Taylor, 2001; Nicoll, 2001; Taber, 1998; Tan & Treagust, 1999), compounds and their formulas (Meşeci, Tekin & Karamustafaoğlu, 2013; Novick & Nussbaum, 1978) and mixtures (Ebenezer & Erickson, 1996; Ebenezer & Fraser, 2001; Lee, O, Eichinger, Anderson, Berkheimer & Blakeslee, 1993; Papageorgiou & Sakka; 2000; Şen & Yılmaz, 2012; Uluçinar Sağır, Tekin & Karamustafaoğlu, 2012; Valanides, 2000).
Cooperative learning has been used in a very limited number of studies to teach all identified science subjects and to overcome learners' misunderstandings (Acar & Tarhan, 2007; Birk & Kurtz, 1999).
It is also very important to prevent learners from forming conceptual misconceptions (Ayas & Özmen, 2002; Bauma, Brant & Sutton, 1990; Griffiths, 1994; Nakhleh, 1992). However, the studies aimed at the prevention or elimination of misconceptions about these issues. In limited studies where extensive misconceptions exist, teaching methods and techniques, other than cooperative learning, are used extensively (Gökharman, 2013; Say, 2011; Uzun, 2010).
From studies carried out in Turkey the effect of cooperative learning on success, attitude, motivation, scientific process skills, self-efficacy, and persistence has been investigated (Bozdoğan, Taşdemir & Demirbaş 2006; Bozkurt, Orhan & Kaynar, 2008; Doğru & Ünlü, 2012; Gençosman, 2011; Tarhan & Sesen, 2012; Tortumluoğlu, 2014; Yapıcı, Hevedanlı & Oral, 2009).
Bozdoğan et al. (2006) examined the relationship between experimental and control group learners' final test scores. The final test scores of the learners in the experimental group were found to be higher than the post-test scores of the learners in the control group. Bozkurt et al. (2008) found that cooperative learning is more effective in increasing the learners' success than traditional learning methods. Doğru and Ünlü (2012) found that there was no relationship between cooperative learning methods and learners' motivation about science lessons. Gençosman (2011) found that cooperative learning has a more significant effect on self-efficacy, academic achievement, and persistence than the traditional teaching method and the current science and technology curriculum, which is based on constructivism. Tarhan and Sesen (2012) found that learners in the experimental group had less misconceptions than learners in the control group. From individual interviews it became clear that learners believed that jigsaw was an effective cooperative learning technique that provides positive attitudes. Tortumluoğlu (2014) determined that there was no significant difference in terms of academic achievement between the groups in which cooperative learning and traditional teaching methods were applied. Yapıcı et al. (2009) found that cooperative learning was more effective in increasing the success levels. When learners' attitudes towards the course were examined, no significant difference was found between the experimental and control groups.
Many studies on cooperative learning as a method with focus on its effects on learners' achievement and attitudes exist in the literature (Ebrahim, 2012; Marzban & Akbernejad, 2013; Nam & Zellner, 2011; Sisovic & Bojovic, 2000; Tarhan & Sesen, 2012; Tran & Lewis, 2012; Zoghi, 2013), while few studies dealing with the effects of cooperative learning on learners' motivation exist (Doğru & Ünlü, 2012; Fernandez-Rio et al., 2017).
Purpose of the Study
The aim of this study was to analyse the effects of instructional curriculum based on cooperative learning (ICBCL) on 7th grade learners' academic achievements, motivations to learn science and their attitudes towards learning science in the subjects, the orders of electrons and chemical properties, chemical bonds, compounds and their formulas, and mixtures. To achieve this purpose the following sub-questions were investigated:
1. What is the effect of ICBCL on learners' achievements about the aforementioned subjects?
2. What is the effect of ICBCL on learners' conceptual misunderstandings?
3. What is the effect of ICBCL on learners' motivation to learn science?
4. What is the effect of ICBCL on learners' attitudes towards learning science?
Methodology
Design
A comparison group pre- and post-test quasi experimental design was used in this study. Participants in the experimental group were taught the instructional curriculum using cooperative learning (ICBCL) while the control group was taught the current Science Teaching Curriculum (STC) used in the relevant academic year. The experimental pattern of the study is presented in Table 1.
Participants
The participants in this study were 89 7th grade learners studying at a public secondary school in Istanbul during the 2013-2014 academic year. Two of the six 7th grade classes were chosen randomly for the implementation of this study. The experimental group was made up of 46 learners while 43 learners were in the control group.
Development of Instructional Curriculum based on Cooperative Learning (ICBCL)
Within the aim of this study, a literature review on the predefined subject matter was done and the learners' misconceptions relating to the aforementioned concepts were determined (Nicoll, 2001; Papageorgiou & Sakka, 2000). The objectives for these subjects were analysed in accordance with Bloom's taxonomy and it was found that the objectives were mainly based on recall and comprehension, which are cognitive stages in Bloom's taxonomies. The objectives were rearranged to also include the cognitive stages of application, analysis, and evaluation. Seven objectives of the Science Teaching Curriculum were not changed, while 14 were changed and nine more added. In order to decide whether these objectives were suitable or not, four science teachers were consulted and 30 objectives, which were analysed according to Bloom's revised taxonomy, were agreed upon.
Cooperative learning tasks were designed in accordance with the objectives of the subjects chosen for this study. Firstly, a literature review on related subjects was done in the task developing process. Current tasks for the different subjects in the 7th grade science book were analysed. The aim of designing new tasks was to allow learners to relate new concepts to the previous ones and engage with these in their daily lives. The results of the analysis done on the related subjects in 7th grade science book show that the tasks did not make it possible for the learners to concretise some of the abstract concepts. For instance, there were no tasks or activities to concretise the concept of the structure of the atom and its particles in the learners' minds. All the activities on the topic of the structure of atoms were to be completed on the paper and none of them required three dimensional work. As a result, within the scope of this study, three dimensional models of atoms were designed in activities like "Let's make an atom model," "Shall we do shopping?," "How do these atoms stand together?" Such activities provided students with opportunities to concretise the concepts of the orders of electrons and chemical properties and chemical bonds.
To determine whether these activities were suitable for the learners' level or not, and to prove that they had content validity, one science teacher and three lecturers were consulted. After making necessary changes based on the experts' opinions, an Activity Book for the Students and an Activity Book for the Teachers were written using compiling all the activities. Each of the activities in the Students' Activity Book was designed in a way that included the required time, safety measures, tools, process steps and evaluation parts. Besides these, the Teachers' Activity Book was designed in a way to include learner objectives, scientific process skills, background information to help the teacher and teacher planning steps needed for the activities. In Table 2, one can see the names of the cooperative learning activities developed for this study and the ICBCL objectives for each activity.
Implementation Process of ICBCL in the Experimental Group
The learning together model which is one of the cooperative learning methods was used in this study. The most important characteristic of this method which was designed by Johnson and Johnson (1999) are that it has an aim, thoughts and materials are shared, there is cooperation and group awards (Açıkgöz, 1992).
The experimental group is made up of 46 learners. Teaching the determined subjects to the experimental group took 4.5 weeks - four lessons per week (one lesson time = 40 minutes) - in accordance with ICBCL. The time used for using the evaluation tests was not included in the implementation process of lessons. One week before the subjects were taught, learners were given DTSMP, MSTLS and ASTSL tests as pre-tests.
Before the learners begin the process of applying the method; the responsibilities of each group member are explained by explaining how to create groups, how to make the task distribution, which responsibilities will be given to the members of the group, which subject will be taught, the rules that the learners and the teacher have to comply within the implementation of the method and the necessary concepts related to the subject.
It is important in the cooperative learning method that small groups of learners should be heterogeneous and the average grades of the groups should be similar or the same (Foyle, Lyman, Morehead & Foyle, 1989). Groups consisting of many learners will be composed of people with different characteristics due to their heterogeneity. For this reason, in groups of few people, learners have the opportunity to encounter a few different ideas, while in the group of many learners, learners will be able to have more different opinions (Köseoğlu & Tümay, 2013).
For this reason, four heterogeneous cooperative groups made up of seven learners and three heterogeneous cooperative groups made up of six learners were formed with the technique of bedded random sampling by using learners' DTSMP test results. After that, the groups were located in a way that allowed the learners in the same group to work together easily and each group works freely and separately from the other groups. Each group was asked to choose a group name to make it possible to have a positive attachment in the group and each learner was given a role as summarizer, reporter, comprehension checker, researcher and encourager and these roles were swapped. For the last thing before the start of the process, the learners were told that they should speak silently and call their friends by their names while working together.
Each group was given an activity set before the activities were started to be done. In this way, the learners were made to share the materials. While the learners were doing the activities, the teacher was going around the classroom, explaining unclear parts and giving leading answers to learners. At the end of the implementation process, randomly chosen learners were asked to summarize the information that they needed to learn. As a result of the feedback gotten from the learners, the teacher diagnosed the information gaps and informed learners about the subject. After the implementation of ICBCL; DTSMP, MSTLS and ASTSL tests were given again as post-tests. A certificate of Participation in Cooperative Learning Activities was given to the learners of the experimental group as an award at the end of the implementation process.
Teaching Process in the Control Group
The control group is made up of 43 learners. In the course of the lessons in the control group, the teacher made a presentation of the same content and similar considering the same learning objectives. In Table 2, one can see the names of activities for this study. Teaching the determined subjects to the control group was done by using the learner textbook and the Teacher's Activity Book. While the teacher explained the issues, the learners listened to her and wrote notes about the issue of the lesson. At the end of the lesson, the teacher had the activities done by the learners in the book. Some activities were given as homework. One week before the subjects were taught, learners were given DTSMP, MSTLS and ASTSL tests as pre-tests. Teaching the subjects synchronously took 4.5 weeks - four lessons per week (one lesson time = 40 minutes). The time used for using the evaluation tests was not included in the implementation process of lessons. At the end of the process, the control group was given DTSMP, MSTLS and ASTSL tests.
At the end of the Application Process of ICBCL in the Experimental Group and the STC in the control group, additional lesson was made by the course teacher to bring the control group to the experimental group level.
Data Collection
Diagnostic test for the structure of matter and its properties (DTSMP)
Within the scope of this study, a two-tier diagnostic test DTSMP which was designed by the researchers was implemented so as to diagnose the experimental and control group learners' level in terms of academic knowledge for the determined subjects. Right after the subject matter was decided on, a literature review was done during the process of test development. An item pool of 53 items was prepared in accordance with the objectives of ICBCL which was designed for this study. A table of specifications was prepared according to Bloom's revised Taxonomy in order to test the construct and content validity of the test. So as to make the test valid in terms of content, experts were consulted and the test was finally decided on 32 items. It was implemented to 225 7th grade learners. The reliability coefficiency of the test was defined as 0.91. The top score that could be gotten from the test was defined as 32.
Motivation scale to learning science (MSTLS)
A Likert type scale MSTLS of 23 items with five choices which was designed by Dede and Yaman (2008) was used before and after the implementation of the study in order to evaluate the motivation of the learners in both experimental and control groups for learning science. Scale: is made up of five dimensions as motivation for searching, motivation for performance, motivation for communication, motivation for cooperative work and motivation for participation. The reliability coefficient of this motivation scale was found to be 0.80 as a result of the reliability analysis done. Answer options for the items of the scale were defined as "5 = Totally Agree," "4 = Agree," "3 = Neutral," "2 = Disagree," "1 = Totally Disagree." The top score that could be gotten from the test was defined as 115.
Attitude scale towards science lesson (ASTSL)
An ASTSL which was designed by Biçer (2011) was used before and after the implementation of the study in order to evaluate the attitudes of the learners in both experimental and control groups about science lessons. The reliability coefficient was 0.898 as a result of the reliability analysis done. The attitude scale is a likert type scale with five choices made up of 11 positive and 15 negative items - 26 items in total. Answer options for the positive items of the scale were defined as "5 = Totally Agree," "4 = Agree," "3 = Partially Agree," "2 = Disagree," "1 = Totally Disagree"; and answer options for the negative items of the scale were defined as "1 = Totally Agree," "2 = Agree," "3 = Partially Agree," "4 = Disagree," "5 = Totally Disagree." The top score that could be gotten from the ASTSL was defined as 130.
Data Analysis
Shapiro-Wilks test was used while analysing the data of this study to decide whether gathered data were ranged equally in both groups because the sample was less than 50 in number (Büyüköztürk, 2007). As a result of the tests, it was considered appropriate to use nonparametric methods of statistics. Programs of SPSS 16 and Lertap 5 were used for data analysis.
Findings
According to the results of the Shapiro-Wilks test, it was seen that the pre-test and post-test points of experimental and control groups from DTSMP, MSTLS and ASTSL tests were not ranged normally (p > 0.05).
The test of DTSMP aiming to answer the question of "What is the effect of ICBCL on learners' achievements about the aforementioned subjects?" was given to the experimental and control groups as pre-test and post-test.
According to the supplementary statistical data of DTSMP, while the average pre-test point of the experimental group was 6.41 for this test, it was 5.88 for the control group. According to the findings of Mann Whitney U-Test, there was not a meaningful difference between the pre-test points of DTSMP of the experimental group and the points of the control group (Table 3). Findings showed that these two groups were equal in the beginning (U = 941.00, p > 0.05).
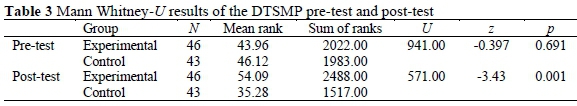
According to the supplementary statistical data of DTSMP, while the average post-test point of the experimental group was 18.35 for this test, it was 12.67 for the control group. As can be seen in Table 3, according to the findings of Mann Whitney U-Test, there was a meaningful difference between the post-test points of DTSMP of the experimental group and the points of the control group (U = 571.00, p < 0.05).
The test of DTSMP aiming to answer the question of "What is the effect of ICBCL on learners' conceptual misunderstandings?" was given to the experimental and control groups as a post-test.
According to the results of the DTSMP, it was showed that learners' in the experimental and control groups have misconceptions. While four of these misconceptions were identified for the first time in this study, 10 of them could be found in the literature. Misconceptions which were identified for the first time: "Atoms containing only eight electrons in the last layer may participate information of links," "When an electron is disconnected from any atom, an electron is disconnected, loses its energy," "A single Cl atom makes only ionic bond with a different atom" and "Salt dissolves homogeneously both in water and oil."
According to the results, while misconceptions ratio of experimental group about "Electron Sequence and Chemical Properties" was between 6-12%, it was 19-26% for the control group, about "Chemical Bonds" was between 4-8%, it was 19-26% for the control group, about "Compounds and Formulas" was between 6-8%, it was 24-26% for the control group and about "Mixtures" was between 4-12%, it was 19-24% for the control group.
The test of MSTLS aiming to answer the question of "What is the effect of ICBCL on learners' motivation to learn science?" was given to the experimental and control groups as pre-test and post-test. According to the supplementary statistical data of MSTLS, while the average pre-test point of the experimental group was 82.78 for this test, it was 80.83 for the control group. According to the findings of Mann Whitney U-Test, there was not a meaningful difference between the pre-test points of MSTLS of the experimental group and the points of the control group (Table 4). Findings showed that these two groups were equal in the beginning (U = 806.500, p > 0.05).
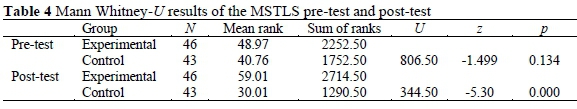
According to the supplementary statistical data of MSTLS, while the average post-test point of the experimental group was 93.13 for this test, it was 75.09 for the control group. As can be seen in Table 4, according to the findings of Mann Whitney U-Test, there was a meaningful difference between the post-test points of MSTLS of the experimental group and the points of the control group (U = 344.50, p < 0.05).
The test of ASTSL aiming to answer the question of "What is the effect of ICBCL on learners' attitudes towards learning science?" was given to the experimental and control groups as pre-test and post-test.
According to the supplementary statistical data of ASTSL, while the average pre-test point of the experimental group was 103.09 for this test, it was 99.51 for the control group. According to the findings of Mann Whitney U-Test, there was not a meaningful difference between the pre-test points of ASTSL of the experimental group and the points of the control group (Table 5). Findings showed that these two groups were equal in the beginning (U = 895.00, p > 0.05).
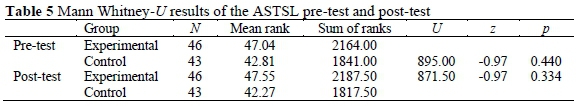
According to the supplementary statistical data of ASTSL, while the average post-test point of the experimental group was 109.17 for this test, it was 105.51 for the control group. As can be seen in Table 5, according to the findings of Mann Whitney U-Test, there was not a meaningful difference between the post-test points of ASTSL of the experimental group and the points of the control group (U = 871.50, p > 0.05).
Discussion and Conclusion
In this study, the effects of ICBCL prepared for the subjects of "the Orders of Electrons and Chemical Properties," "Chemical Bonds," "Compounds and Their Formulas," "Mixtures" of 7th Grade Science class on the learners' academic achievement, motivation to learn science and their attitudes towards learning science were analysed. For this aim, the lessons were done by using ICBCL in the experimental group and lessons were done with STC of related academic year in the control group.
As a result of the DTSMP post-test, the experimental group was found to be more successful when compared to the control group at the end of the implementation process. This means that ICBCL is more efficient in teaching the determined subjects when compared to STC. Local and international studies also show that teaching subjects by using cooperative learning method increase the success rate of the learners as in the results of this study. Ebrahim (2012) and Ergün (2006) also proved that teaching subjects by using the cooperative learning method increased the success rate of their 8th grade learners. Besides, it shows the results of different researchers' cooperative learning method is highly influential on the success of learners (Zoghi, 2013). Improved classroom learning environments should encourage improved academic scientific achievement (Schulze & Van Heerden, 2015).
According to the results of the DTSMP, learners in the experimental group had significantly fewer misconceptions than learners in the control group. These difficulties were categorized as misconceptions related to electron sequence and chemical properties (Stable Atom, Ion and Atom Model), Chemical Bonds (Cation and Anion, Ionic Bonds and Covalent Bonds), Compounds and Formulas (Compound, Ionic and Molecular Compound) and Mixtures (Mixture, Dissolution Event, Dissolution Rate and Conductivity Solution). It was determined that some learners in the control group had misconceptions related to Electron sequence and chemical properties concept such as "Atoms containing only eight electrons in the last layer may participate information of links" and "When an electron is disconnected from any atom, an electron is disconnected, loses its energy." These two misconceptions were first identified in the context of this study. Also learners had misconceptions about the "Atom Model" concept such as "For an atom, to be chemically determined, it has to have two electrons in its outmost energy level." Similar findings had been reported by Harrison and Treagust (2000) and Kara and Ergül (2012). It was determined that some learners in the control group had misconceptions related to Chemical Bonds concepts such as "Atoms become anion by accepting proton, and become cation by giving proton" and "A single Cl atom makes only ionic bond with a different atom." This misconception was first identified in the context of this study. Learners also had misconceptions about "Covalent Bonds" concept such as "Salt (NaCI) and water (H2O), both have covalent structure." The similar alternative conceptions had been reported before by Nicoll (2001). It was determined that some learners in the control group had misconceptions related to Compounds and Formulas concepts such as "Compounds are divided into components physically." The results of different researchers also show similar results (Meşeci et al., 2013). It was determined that some learners in the control group had misconceptions related to Mixtures concepts such as "Salt dissolves homogeneously both in water and oil." This misconception was firstly identified in the context of this study. Learners also had misconceptions about "Salty water is a heterogeneous mixture." Similar findings had been reported by Milenković, Hrin, Segedinac and Horvat (2016). Some misconceptions are about "Dissolution Event" concept as "Salt, melts in water," "Disappearing." The results of different researchers also show similar results (Demirbaş & Ertuğrul, 2014; Lee, O et al., 1993; Uzun, 2010). The misconceptions determined in the result of this research are shown that learners could not establish relationships between concepts and had difficulty in structuring information as a result learners cannot perform effective learning. It is very important to the development and implementation of the curriculum in which learners are active in the learning process. The cooperative learning method based on the constructivist approach is applied in learning science topics (Ebrahim, 2012).
MSTLS which was given as a post-test at the end of the implementation process showed that the motivation level of the experimental group towards science dramatically increased when compared to the control group. This means that ICBCL is effective in increasing learners' interest in Science lessons and their motivation. Local and international studies also have similar results with the results of this study. It was found that using cooperative learning method increased the interest and motivation of the learners in their related studies of "Science Lesson" by Oh and Shin (2005), "Biology" by Keraro, Wachanga and Orora (2007); "Power and Motion" by Doğan, Uygur, Doymuş and Karaçöp (2010). Along with this, it was seen that there were a few studies concluding that the cooperative learning method had nothing to do with the learners' motivation. Doğru and Ünlü (2012) found that there was no relationship between the cooperative learning method and learners' motivation about Science lessons.
ASTSL which was given to experimental and control groups as a post-test at the end of the implementation process showed that although there was an increase in the attitude level of experimental group towards Science lesson, the difference was not found to be meaningful. The results which were gotten from the studies named "A Journey to the Inner Structure of Matter" by Demiral (2007), and "Mixtures" by Genç (2007) are in parallel with the results of this study. Besides, it shows the results of different researchers cooperative learning method did not significantly change the attitudes of learners (Umdu Topsakal, 2010). However, in their related studies, Ballıel (2014) and Lowe (2004) found that there was a meaningful difference between the learners' attitudes towards Science lessons as opposed to the results of this study.
In addition to the teaching methods used in the effective realization of learning, affective field features also play an important role. One of the affective field characteristics is attitude. In the measurement of the affective field characteristics, it is not possible to observe these features directly and thus indirect measurement is used. For this reason, either long-term observation is made or the learner is interacted with artificial situations to determine how he tends to behave in these situations (Genç & Şahin, 2015). Zacharia and Barton (2004) stated that the attitude from affective field behaviors is resistant to time, that it is a phenomenon that can change with personal beliefs, and that it can be learned over time in the process (Zacharia & Barton, 2004). It has also been shown in different studies in the literature that the attitude changed over time (Baykul, 2004).
The reason why there was not a meaningful difference between the experimental group and the control group can be that the time used to apply ICBCL was limited, the learners were not used to work as a group while doing a task, they needed more time to get used to the method, they were used to get information readily by their teachers and that they found it difficult to work individually.
Suggestions as a result of the findings of this study: Further ICBCL for the other topics of Science lessons can be developed. Further curriculums for the other subjects can be developed by taking ICBCL as an example. The efficiency and practicality of this curriculum can be researched by doing similar studies about ICBCL for the other grades. The dependent variables whose effects on the experimental group who were taught with ICBCL were analysed on academic achievement, motivation and the learners' level of attitude towards Science lesson. Apart from these variables; further studies on the variables like critical thinking, creative thinking, empathetic thinking, logical thinking and self sufficiency can also be done.
Advanced economies, innovative industries and firms, and high-growth jobs require more educated workers with the ability to respond flexibly to complex problems, communicate effectively, manage information, work in teams and produce new knowledge. For this reason, individuals especially in developing countries such as India, Philippines, Thailand, Turkey, South Africa, Brazil must have 21st century skills. As shown in the results of this study, cooperative learning has the potential to train individuals who have those skills.
Acknowledgements
Thank you to the scientific research fund of Istanbul University for the financial support provided for this study with the project no: 22921.
Authors' Contributions
FA was responsible for data collection and the first draft manuscript; FGK and BAS contributed to the conceptualisation of the study, the analysis and writing of the manuscript. All authors reviewed the final manuscript.
Notes
i. Published under a Creative Commons Attribution Licence.
ii. DATES: Received: 27 September 2017; Revised: 4 December 2018; Accepted: 27 April 2019; Published: 31 August 2019.
References
Acar B & Tarhan L 2007. Effect of cooperative learning strategies on students' understanding of concepts in electrochemistry. International Journal of Science and Mathematics Education, 5(2):349-373. https://doi.org/10.1007/s10763-006-9046-7 [ Links ]
Açıkgöz KÜ 1992. İşbirlikli öğrenme: Kuram, araştırma, uygulama [Cooperative learning: Theory, research, practice]. Malatya, Turkey: Uğurel Matbaası [ Links ].
Aldoobie N 2015. ADDIE model. American International Journal of Contemporary Research, 5(6):68-72. Available at http://www.aijcrnet.com/journals/Vol_5_No_6_December_2015/10.pdf. Accessed 18 August 2019. [ Links ]
Arkün S 2007. ADDIE tasarım modeline göre çoklu öğrenme ortamı geliştirme süreci ve geliştirilen ortam hakkında öğrenci görüşleri üzerine bir çalışma [A study on student learning about the multiple learning environment development process and environment developed according to the ADDIE design model]. Master's thesis. Ankara, Turkey: Hacettepe University. [ Links ]
Aruna PK & Sumi VS 2010. Process approach: Effect on attitude towards science process skills in science. Journal of All India Association for Educational Research, 22(1):76-81. [ Links ]
Ayas A & Özmen H 2002. Lise kimya öğrencilerinin maddenin tanecikli yapısı kavramını anlama seviyelerine ilişkin bir çalışma [A study of students' level of understanding of the particulate nature of matter at secondary school level]. Boğaziçi Üniversitesi Eğitim Dergisi, 19(2):45-60. Available at https://chemistrynetwork.pixel-online.org/data/SMO_db/doc/57_2.pdf. Accessed 16 August 2019. [ Links ]
Ballıel B 2014. Webquest destekli işbirlikli öğrenme yaklaşımının öğrenme ürünlerine etkisi [Impact of Webquest supported collaborative learning approach on learning products]. PhD thesis. Ankara, Turkey: Gazi University. [ Links ]
Bauma H, Brant I & Sutton C 1990. Words as tools in science lessons: Chemiedidactiek. Amsterdam, Netherlands: University of Amsterdam. [ Links ]
Baykul Y 2004. İlköğretim matematik öğretimi (6-8. Sınıflar için) [Primary mathematics teaching (for grades 6-8)]. Ankara, Turkey: Pegem Akademi Yayıncılık. [ Links ]
Biçer S 2011. Fen ve teknoloji dersinde basamaklı öğretim yönteminin öğrenci başarısına, kalıcılığa ve tutumlarına etkisi [The impact of step-by-step instruction in science and technology in student achievement, retention and attitudes]. Master's thesis. Elazığ, Turkey: Fırat Üniversitesi. Available at https://openaccess.firat.edu.tr/xmlui/bitstream/handle/11508/12468/289694.pdf?sequence=1. Accessed 14 August 2019. [ Links ]
Birk JP & Kurtz MJ 1999. Effect of experience on retention and elimination of misconceptions about molecular structure and bonding. Journal of Chemical Education, 76(1):124-128. https://doi.org/10.1021/ed076p124 [ Links ]
Bozdoğan AE, Taşdemir A & Demirbaş M 2006. Fen bilgisi öğretiminde işbirlikli öğrenme yönteminin öğrencilerin bilimsel süreç becerilerini geliştirmeye yönelik etkisi [The effect of cooperative learning method in science education on improving the students'science process skills]. Eğitim Fakültesi Dergisi, 7(11):23-36. Available at https://www.researchgate.net/publication/294715809_Fen_bilgisi_ogretiminde_isbirlikli_ogrenme_yonteminin_ogrencilerin_bilimsel_surec_becerilerini_gelistirmeye_yonelik_etkisi. Accessed 14 August 2019. [ Links ]
Bozkurt O, Orhan AT & Kaynar G 2008. Fen ve teknoloji laboratuarı uygulamaları I-II [Science and technology laboratory applications I-II]. Ankara, Turkey: Maya Akademi Yayıncılık. [ Links ]
Butts B & Smith R 1987. HSC chemistry students' understanding of the structure and properties of molecular and ionic compounds. Research in Science Education, 17(1):192-201. https://doi.org/10.1007/BF02357187 [ Links ]
Büyüköztürk Ş 2007. Sosyal bilimler için very analizi el kitabı [Data analysis handbook for social sciences]. Baskı. Ankara, Turkey: Pegem Akademi Yayıncılık. [ Links ]
Carpenter S & McMillan T 2003. Incorporation of a cooperative learning technique in organic chemistry. Journal of Chemical Education, 80(3):330-332. https://doi.org/10.1021/ed080p330 [ Links ]
Cheung L 2016. Using the ADDIE model of instructional design to teach chest radiograph interpretation. Journal of Biomedical Education, 2016:1-6. https://doi.org/10.1155/2016/9502572 [ Links ]
Coll RK & Taylor N 2001. Alternative conceptions of chemical bonding held by upper secondary and tertiary students. Research in Science & Technological Education, 19(2):171-191. https://doi.org/10.1080/02635140120057713 [ Links ]
Dede Y & Yaman S 2008. Fen öğrenmeye yönelik motivasyon ölçeği: Geçerlik ve güvenirlik çalışması [A questionnaire for motivation towrad science learning: A validity and reliability study]. Necatibey Eğitim Fakültesi Elektronik Fen ve Matematik Eğitimi Dergisi (EFMED) [Necatibey Faculty of Education Electronic Journal of Science and Mathematics Education], 2(1):19-37. Available at https://dergipark.org.tr/download/article-file/39760. Accessed 6 August 2019. [ Links ]
Demiral S 2007. İlköğretim fen bilgisi dersi maddenin iç yapısına yolculuk ünitesinde, işbirlikli öğrenme yönteminin öğrenci başarısına bilgilerin kalıcılığına ve derse karşı tutumlarına etkisi [The effect of the cooperative learning method on student achievement, persistence of knowledge and attitudes towards the lesson in the elementary science students' journey to Madden's inside journey unit]. Master's thesis. Ankara, Turkey: Gazi University. [ Links ]
Demirbaş M & Ertuğrul N 2014. A study on preschoolers' conceptual perceptions of states of matter: A case study of Turkish students. South African Journal of Education, 34(3):Art. # 920, 13 pages. https://doi.org/10.15700/201409161115 [ Links ]
Doğan A, Uygur E, Doymuş K & Karaçöp A 2010. İlköğretim 7. sınıf Fen ve Teknoloji dersinde Jigsaw tekniğinin uygulanması ve bu teknik hakkındaki öğrenci görüşleri [The use of Jigsaw technique in 7th grade primary science and technology course and students'views on this technique]. Erzincan Eğitim Fakültesi Dergisi, 12(1):75-90. Available at https://arastirmax.com/en/system/files/dergiler/5671/makaleler/12/1/arastirmax-ilkogretim-7.sinif-fen-teknoloji-dersinde-jigsaw-tekniginin-uygulanmasi-bu-teknik-hakkindaki-ogrenci-gorusleri.pdf. Accessed 3 August 2019. [ Links ]
Doğru M & Ünlü S 2012. Jigsaw IV tekniği kullanımının fen öğretiminde öğrencilerin motivasyon, fen kaygısı ve akademik başarılarına etkisi [The effects of employing the Jigsaw IV technique in science and technology: Education upon students' motivation, science anxiety and their academic achievement]. Mediterranean Journal of Humanities, II(2):57-66. https://doi.org/10.13114/MJH/20122738 [ Links ]
Ebenezer JV & Erickson GL 1996. Chemistry students' conception of solubility: A phenomenography. Science Education, 80(2):181-201. https://doi.org/10.1002/(SICI)1098-237X(199604)80:2%3C181::AID-SCE4%3E3.0.CO;2-C [ Links ]
Ebenezer JV & Fraser DM 2001. First year chemical engineering students' conception of energy in solution processes: Phenomenographic categories for common knowledge construction. Science Education, 85(5):509-535. https://doi.org/10.1002/sce.1021 [ Links ]
Ebrahim A 2012. The effect of cooperative learning strategies on elementary students' science achievement and social skills in Kuwait. International Journal of Science and Mathematics Education, 10(2):293-314. https://doi.org/10.1007/s10763-011-9293-0 [ Links ]
Erden M 1993. Eğitimde program değerlendirme [Curriculum evaluation in education]. Ankara, Turkey: Pegem Yayınları [ Links ].
Ergün A 2006. İşbirlikli öğrenme yönteminin ilköğretim sekizinci sınıf fen öğretimine etkileri [Effects of Cooperative Learning Method on elementary school science teaching in eighth grade]. Master's thesis. Denizli, Turkey: Pamukkale University. [ Links ]
Fernandez-Rio J, Sanz N, Fernandez-Cando J & Santos L 2017. Impact of a sustained cooperative learning intervention on student motivation. Physical Education and Sport Pedagogy, 22(1):89-105. https://doi.org/10.1080/17408989.2015.1123238 [ Links ]
Foyle HC, Lyman L, Morehead AM & Foyle JC 1989. Interactive learning: Creating an environment for cooperative learning. Paper presented at the 44th Annual Conference of the Association for Supervision and Curriculum Development (ASCD), Orlando, FL, 13 March. Available at https://files.eric.ed.gov/fulltext/ED305335.pdf. Accessed 23 August 2019. [ Links ]
Genç M 2007. İşbirlikli öğrenmenin problem çözmeye ve başarıya etkisi [The effect of cooperative learning on problem solving and success]. PhD thesis. Istanbul, Turkey: Marmara University. Available at https://www.researchgate.net/profile/Murat_Genc4/publication/284124200_Isbirlikli_ogrenmenin_problem_cozmeye_ve_basariya_etkisi/links/56fbcaf408aef6d10d91b31b/Isbirlikli-oegrenmenin-problem-coezmeye-ve-basariya-etkisi.pdf. Accessed 1 August 2019. [ Links ]
Genç M & Şahin F 2015. İşbirlikli öğrenmenin başarıya ve tutuma etkisi [The effects of cooperative learning on attitude and achievement]. Necatibey Eğitim Fakültesi Elektronik Fen ve Matematik Eğitimi Dergisi (EFMED) [Necatibey Faculty of Education Electronic Journal of Science and Mathematics Education], 9(1):375-396. Available at https://dergipark.org.tr/download/article-file/39925. Accessed 1 August 2019. [ Links ]
Gençosman T 2011. The use of student teams' success parts technique in science and technology teaching influences students' self-efficacy, test anxiety, academic achievement and remembrance levels. Master's thesis. Antalya, Turkey: Akdeniz University. [ Links ]
Genlott AA & Grönlund Å 2013. Improving literacy skills through learning reading by writing: The iWTR method presented and tested. Computers & Education, 67:98-104. https://doi.org/10.1016/j.compedu.2013.03.007 [ Links ]
Gilbert JK, Justi R, Van Driel JH, De Jong O & Treagust DF 2004. Securing a future for chemical education. Chemistry Education: Research and Practice, 5(1):5-14. https://doi.org/10.1039/B3RP90027D [ Links ]
Gillies RM 2006. Teachers' and students' verbal behaviours during cooperative and small-group learning. British Journal of Educational Psychology, 76(2):271-287. https://doi.org/10.1348/000709905X52337 [ Links ]
Gökharman HK 2013. Maddenin yapısı ve özellikleri ünitesinde analoji kullanımının öğrenci başarısına ve tutumuna etkisi (Çivril örneği) [The effect of the use of analogy on the structure and properties of matter on student success and attitude (Çivril example)]. Master's thesis. Denizli, Turkey: Pamukkale University. [ Links ]
Griffiths AK 1994. A critical analysis and synthesis of research on chemistry misconceptions. In HJ Schmidt (ed). Proceedings of the 1994 International Symposium on Problem Solving and Misconceptions in Chemistry and Physics. Dortmund, Germany: The International Council of Associations for Science Education (ICASE). [ Links ]
Griffiths AK & Preston KR 1992. Grade-12 students' misconceptions relating to fundamental characteristics of atoms and molecules. Journal of Research in Science Teaching, 29(6):611-628. https://doi.org/10.1002/tea.3660290609 [ Links ]
Harrison AG & Treagust DF 2000. Learning about atoms, molecules, and chemical bonds: A case study of multiple model use in grade 11 chemistry. Science Education, 84(3):352-381. https://doi.org/10.1002/(SICI)1098-237X(200005)84:3%3C352::AID-SCE3%3E3.0.CO;2-J [ Links ]
Harrison AG & Treagust DF 2003. The particulate nature of matter: Challenges in understanding the submicroscopic world. In JK Gilbert, O de Jong, R Justi, DF Treagust & JH van Driel (eds). Chemical education: Towards research-based practice. Dordrecht, The Netherlands: Springer. https://doi.org/10.1007/0-306-47977-X [ Links ]
Jacobs GM 1990. Foundations of cooperative learning. Paper presented at the Annual Meeting of the Hawai Educational Research Association, Honolulu, HI, 9 January. Available at https://files.eric.ed.gov/fulltext/ED351363.pdf. Accessed 24 August 2019. [ Links ]
Johnson DW & Johnson RT 1999. Learning together and alone: Cooperative, competitive, and individualistic learning (5th ed). Boston, MA: Allyn & Bacon. [ Links ]
Johnson DW, Johnson RT & Smith K 2007. The state of cooperative learning in postsecondary and professional settings. Educational Psychology Review, 19(1):15-29. https://doi.org/10.1007/s10648-006-9038-8 [ Links ]
Johnstone AH 1993. The development of chemistry teaching: A changing response to changing demand. Journal of Chemical Education, 70(9):701-705. https://doi.org/10.1021/ed070p701 [ Links ]
Jonassen DH 1991. Objectivism versus constructivism: Do we need a new philosophical padarigm? Education Technology Research and Development, 39(3):5-14. https://doi.org/10.1007/BF02296434 [ Links ]
Kaminski J 2007. Use ADDIE to design online courses. Available at https://nursing-informatics.com/ADDIE.pdf. Accessed 25 May 2016. [ Links ]
Kara F & Ergül S 2012. Fen bilgisi öğretmen adaylarının çözünme ile ilgili temel kavramlar hakkındaki bilgilerinin incelenmesi [Examination of the basic knowledge about dissolution of pre-service science teachers]. Eğitim ve Öğretim Araştırmaları Dergisi [Journal of Research in Education and Teaching], 1(2):2146-9199. Available at http://www.jret.org/FileUpload/ks281142/File/29._kara.pdf. Accessed 26 July 2019. [ Links ]
Kauchak D & Eggen P 2003. Learning and teaching: Research-based methods (4th ed). Boston, MA: Pearson. [ Links ]
Keraro FN, Wachanga SW & Orora W 2007. Effects of cooperative concept mapping teaching approach on secondary school students' motivation in biology in Gucha District, Kenya. International Journal of Science and Mathematics Education, 5(1):111-124. https://doi.org/10.1007/s10763-005-9026-3 [ Links ]
Köseoğlu F, Atasoy B, Kavak N, Akkuş H, Budak E, Tümay H, Kadayıfçı H & Taşdelen U 2003. Yapılandırıcı öğrenme ortamı için bir fen ders kitabı nasıl olmalı [How to be a science textbook for a constructivist learning environment]. Ankara, Turkey: Asil Yayın Dağıtım Ltd. [ Links ]
Köseoğlu F & Tümay H 2013. Bilim eğitiminde yapılandırıcı paradigma: Teoriden öğretim uygulamalarına [Constructive paradigm in science education: From theory to teaching practices]. Ankara, Turkey: Pegem Akademi. [ Links ]
Lazarowitz R 1991. Learning biology cooperatively: An Israeli junior high school study. Cooperative Learning, 11(3):19-21. [ Links ]
Lee J & Jang S 2014. A methodological framework for instructional design model development: Critical dimensions and synthesized procedures. Educational Technology Research and Development, 62(6):743-765. https://doi.org/10.1007/s11423-014-9352-7 [ Links ]
Lee O, Eichinger DC, Anderson CW, Berkheimer GD & Blakeslee TD 1993. Changing middle school students' conceptions of matter and molecules. Journal of Research in Science Teaching, 30(3):249-270. https://doi.org/10.1002/tea.3660300304 [ Links ]
Liao HC 2006. Effects of cooperatıve learning on motivation, learning strategy utilizatıon, and grammar achievement of Englısh language learners in Taiwan. PhD dissertation. New Orleans, LA: University of New Orleans. Available at https://scholarworks.uno.edu/cgi/viewcontent.cgi?referer=https://scholar.google.com/&httpsredir=1&article=1362&context=td. Accessed 25 July 2019. [ Links ]
Lowe JP 2004. The effect of cooperative group work and assessment on the attitudes of students towards science in New Zealand. PhD thesis. Perth, Australia: Curtin University of Technology. Available at https://espace.curtin.edu.au/bitstream/handle/20.500.11937/955/15358_Lowe,%20John%20Paul%202004.pdf?sequence=2&isAllowed=y. Accessed 25 July 2019. [ Links ]
Marzban A & Akbarnejad AA 2013. The effect of cooperative reading strategies on improving reading comprehension of Iranian university students. Procedia - Social and Behavioral Sciences, 70:936-942. https://doi.org/10.1016/j.sbspro.2013.01.141 [ Links ]
Merritt JD, Shwartz Y & Krajcik J 2007. Middle school students' development of the particle model of matter. Paper presented at the annual meeting of the National Association of Research in Science Teaching, New Orleans, LA, April. Available at http://www.umich.edu/~hiceweb/PDFs/2007/Merritt%20et%20al%20NARST%202007.pdf. Accessed 26 August 2019. [ Links ]
Meşeci B, Tekin S & Karamustafaoğlu S 2013. Maddenin tanecikli yapisiyla ilgili kavram yanilgilarinin tespiti [Determination of misconceptions about the particle structure of matter]. Dicle Üniversitesi Sosyal Bilimler Enstitüsü Dergisi, 5(9):20-40. Available at http://www.e-dusbed.com/Dusbed/ArchiveIssues/PDF/5888f45d-6868-e711-80f0-00224d68272d. Accessed 25 July 2019. [ Links ]
Milenković DD, Hrin TN, Segedinac MD & Horvat S 2016. Identification of misconceptions through multiple choice tasks at municipal Chemistry competition test. Journal of Subject Didactics, 1(1):3-12. https://doi.org/10.5281/zenodo.55468 [ Links ]
Nakhleh MB 1992. Why some students don't learn chemistry: Chemical misconceptions. Journal of Chemical Education, 69(3):191-196. https://doi.org/10.1021/ed069p191 [ Links ]
Nam CW & Zellner RD 2011. The relative effects of positive interdependence and group processing on student achievement and attitude in online cooperative learning. Computers & Education, 56(3):680-688. https://doi.org/10.1016/j.compedu.2010.10.010 [ Links ]
Nicoll G 2001. A report of undergraduates' bonding misconceptions. International Journal of Science Education, 23(7):707-730. https://doi.org/10.1080/09500690010025012 [ Links ]
Novick S & Nussbaum J 1978. Junior high school pupils' understanding of the particulate nature of matter. An interview study. Science Education, 62(3):273-281. https://doi.org/10.1002/sce.3730620303 [ Links ]
Oh PS & Shin MK 2005. Students' reflections on the implementation of group investigation in Korean secondary science classrooms. International Journal of Science and Mathematics Education, 3(2):327-349. https://doi.org/10.1007/s10763-004-4502-8 [ Links ]
Okumuş S, Çavdar O, Alyar M & Doymuş K 2017. Kimyasal denge konusunun mikro boyutta anlaşılmasına farklı öğretim yöntemlerinin etkisi [The effect of different teaching methods to understanding of chemical equilibrium at micro level]. İlköğretim online [Elementary Education Online],16(2):727-745. https://doi.org/10.17051/ilkonline.2017.304730 [ Links ]
Özerbaş MA & Kaya AB 2017. Öğretim tasarımı çalışmalarının içerik analizi: ADDIE modeli örneklemi. Türk Eğitim Bilimleri Dergisi, 15(1):26-42. Available at https://dergipark.org.tr/download/article-file/314879. Accessed 22 July 2019. [ Links ]
Papageorgiou G & Sakka D 2000. Primary school teachers' views on fundamental chemical concepts. Chemistry Education Research and Practice, 1:237-247. https://doi.org/10.1039/A9RP90025J [ Links ]
Partnership for 21st Century Skills 2008. 21st century skills, education and competitiveness: A resource and policy guide. Tucson, AZ: Author. Available at https://files.eric.ed.gov/fulltext/ED519337.pdf. Accessed 27 August 2019. [ Links ]
Peterson C 2003. Bringing ADDIE to life: Instructional design at its best. Journal of Educational Multimedia and Hypermedia, 12(3):227-241. Available at https://www.learntechlib.org/p/2074/. Accessed 22 July 2019. [ Links ]
Reigeluth CM 1999. What is instructional-design theoryand how is it changing? In CM Reigeluth (ed). Instructional-design theories and models: A new paradigm of instructional theory (Vol. 2). Mahwah, NJ: Lawrence Erlbaum Associates. [ Links ]
Reinbold S 2013. Using the ADDIE model in designing library instruction. Medical Reference Services Quarterly, 32(3):244-256. https://doi.org/10.1080/02763869.2013.806859 [ Links ]
Saban A 2004. Öğrenme-öğretme süreci :Yeni teori ve yaklaşımlar [Learning-teaching process: New theories and approaches] (3rd ed). Ankara, Turkey: Nobel Yayın Dağıtım. [ Links ]
Say FS 2011. Kavram karikatürlerinin 7. sınıf öğrencilerinin "madenin yapısı ve özelikleri" konusunu öğrenmelerine etkisi [The effect of concept cartoons on 7th grade students to learn the structure of matter and properties]. Master's thesis. Trabzon, Turkey: Karadeniz Technical University. [ Links ]
Schulze S & Van Heerden M 2015. Learning environments matter: Identifying influences on the motivation to learn science. South African Journal of Education, 35(2):Art. # 1058, 9 pages. https://doi.org/10.15700/saje.v35n2a1058 [ Links ]
Şen Ş & Yılmaz A 2012. Erime ve çözünmeyle ilgili kavram yanılgılarının ontoloji temelinde incelenmesi [Examination of misconceptions related to melting and dissolution on the basis of ontology]. Amasya Üniversitesi Eğitim Fakültesi Dergisi, 1(1):54-72. Available at https://dergipark.org.tr/download/article-file/19589. Accessed 18 July 2019. [ Links ]
Siegel C 2005. Implementing a research-based model of cooperative learning. The Journal of Educational Research, 98(6):339-349. https://doi.org/10.3200/JOER.98.6.339-349 [ Links ]
Sisovic D & Bojovic S 2000. Approaching the concepts of acids and bases by cooperative learning. Chemistry Education: Research and Practice in Europe, 1:263-275. https://doi.org/10.1039/A9RP90027F [ Links ]
Slavin RE 1995. Cooperative learning: Theory, research and practice (2nd ed). Boston, MA: Allyn and Bacon. [ Links ]
Taber KS 1998. An alternative conceptual framework from chemistry education. International Journal of Science Education, 20(5):597-608. https://doi.org/10.1080/0950069980200507 [ Links ]
Tan KCD & Treagust DF 1999. Evaluating students' understanding of chemical bonding. School Science Review, 81(294):75-84. Available at https://repository.nie.edu.sg/bitstream/10497/14150/1/SSR-81-294-75.pdf. Accessed 15 July 2019. [ Links ]
Tarhan L & Sesen BA 2012. Jigsaw cooperative learning: Acid-base theories. Chemistry Education Research and Practice, 13:307-313. https://doi.org/10.1039/C2RP90004A [ Links ]
Tatar N & Cansüngü Koray Ö 2005. İlköğretim sekizinci sınıf öğrencilerinin "genetik" ünitesi hakkındaki kavram yanılgılarının belirlenmesi [Determination of the misconceptions of the 8th grade students in primary school about "genetics" unit]. Kastamonu Eğitim Dergisi, 13(2):415-426. Available at https://docplayer.biz.tr/21942398-Ilkogretim-sekizinci-sinif-ogrencilerinin-genetik-unitesi-hakkindaki-kavram-yanilgilarinin-belirlenmesi.html. Accessed 26 July 2019. [ Links ]
Tortumluoğlu Y 2014. İşbirlikli öğrenme modelinin fen ve teknoloji dersinde öğrenci başarısına etkisi: Ardahan ili örneği [The effect of cooperative learning model on student achievement in science and technology class: Cade of Ardahan]. Master's thesis. Erzurum, Turkey: Ataturk University. [ Links ]
Tran VD & Lewis R 2012. The effects of jigsaw learning on students'attitudes in a Vietnamese higher education classroom. International Journal of Higher Education, 1(2):9-20. https://doi.org/10.5430/ijhe.v1n2p9 [ Links ]
Uluçinar Sağır Ş, Tekin S & Karamustafaoğlu S 2012. Sınıf öğretmeni adaylarinin bazi kimya kavramlarini anlama düzeleri [The levels of prospective elementary school taechers' understanding of some chemistry concepts]. Dicle Üniversitesi Ziya Gökalp Eğitim Fakültesi Dergisi, 19:112-135. Available at http://www.zgefdergi.com/Makaleler/750556339_19_09_ID_263.pdf. Accessed 19 July 2019. [ Links ]
Umdu Topsakal U 2010. 8. Sınıf 'canlılar için madde ve enerji' ünitesi öğretiminde işbirlikli öğrenme yönteminin öğrenci başarısına ve tutumuna etkisi [The effectiveness of cooperative learning on teaching 8th class unit 'substance and energy for living things']. Ahi Evran Üniversitesi Eğitim Fakültesi Dergisi, 11(1):91-104. Available at http://kefad.ahievran.edu.tr/InstitutionArchiveFiles/f44778c7-ad4a-e711-80ef-00224d68272d/d1a3a581-af4a-e711-80ef-00224d68272d/Cilt11Sayi1/JKEF_11_1_2010_91_104.pdf. Accessed 13 July 2019. [ Links ]
Uzun B 2010. Fen ve teknoloji öğretiminde kavramsal değişim stratejilerine dayalı olarak maddenin yapısı ve özellikleri konusunun öğretimi. PhD thesis. Izmir, Turkey: Dokuz Eylul University. Available at http://acikerisim.deu.edu.tr/xmlui/handle/20.500.12397/6801. Accessed 28 August 2019. [ Links ]
Valanides N 2000. Primary student teachers' understanding of the particulate nature of matter and its transformations during dissolving. Chemistry Education Research and Practice, 1:249-262. https://doi.org/10.1039/A9RP90026H [ Links ]
Veenman S, Van Benthum N, Bootsma D, Van Dieren J & Van der Kemp N 2002. Cooperative learning and teacher education. Teaching and Teacher Education, 18(1):87-103. https://doi.org/10.1016/S0742-051X(01)00052-X [ Links ]
Yang E, Andre T, Greenbowe TJ & Tibell L 2003. Spatial ability and the impact of visualization/animation on learning electrochemistry. International Journal of Science Education, 25(3):329-349. [ Links ]
Yapıcı İÜ, Hevedanlı M & Oral B 2009. İşbirlikli öğrenme ve geleneksel öğretim tohumlu bitkiler sistematiği laboratuarı dersine yönelik tutum ve başarıya etkisi [The effect of cooperative learning and traditional teaching methods on students' attitudes and achievement in systematic of seed plants laboratory course]. Pamukkale Üniversitesi Eğitim Fakültesi Dergisi, 26:63-69. Available at http://pauegitimdergi.pau.edu.tr/Makaleler/897793002_%c4%b0.%c3%9cmit%20YAPICI1%20Murat%20HEVEDANLI2%20Beh%c3%a7et%20ORAL3.pdf. Accessed 31 August 2019. [ Links ]
Zacharia Z & Barton AC 2004. Urban middle-school students' attitudes toward a defined science. Science Education, 88(2):197-222. https://doi.org/10.1002/sce.10110 [ Links ]
Zoghi M 2013. Let's cross the rubicon: Strengthening reading comprehension instruction. Procedia - Social and Behavioral Sciences, 70:537-543. https://doi.org/10.1016/j.sbspro.2013.01.091 [ Links ]














