Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Surgery
On-line version ISSN 2078-5151
Print version ISSN 0038-2361
S. Afr. j. surg. vol.62 n.1 Cape Town 2024
http://dx.doi.org/10.36303/SAJS.00201
CASE REPORT
Gestational gigantomastia complicated by pseudo-angiomatous stromal hyperplasia – a multidisciplinary management approach
FH RabeI; M ConradieII; M MahokoIII; RC de VilliersIV; J EdgeIV
IStellenbosch University, South Africa
IIDivision of Endocrinology, Department of Medicine, Faculty of Medicine and Health Sciences, Tygerberg Academic Hospital, Stellenbosch University, South Africa
IIIDivision of Plastic and Reconstructive Surgery, Department of Surgery, Faculty of Medicine and Health Sciences, Tygerberg Academic Hospital, Stellenbosch University, South Africa
IVBreast and Endocrine Division, Department of Surgery, Faculty of Medicine and Health Sciences, Tygerberg Academic Hospital, Stellenbosch University, South Africa
SUMMARY
Gestational gigantomastia is a rare condition typified by disproportionate bilateral breast enlargement in pregnant women, resulting in skin thinning, ulceration, and bleeding. Less than sixty cases have been documented worldwide, and only one other in South Africa. Pseudo-angiomatous stromal hyperplasia (PASH) is a rare benign proliferation of stromal tissue in a tumorous or diffuse pattern. This, to the best of our knowledge, is the first published case, a 27-year-old human immunodeficiency virus (HIV) positive woman, to present with both conditions concurrently. Medical management with cabergoline was initiated and, seven months post-delivery, a novel Goldilocks mastectomy was performed with acceptable outcomes.
Keywords: gestational gigantomastia, macromastia, pseudo-angiomatous stromal hyperplasia, Goldilocks mastectomy
Case
A 27-year-old HIV-positive female presented at 16 weeks gestation complaining of massive bilateral breast enlargement, which had started at four weeks gestation, initially from localised foci, and later, diffusely. She also complained of persistent pressure-like pain on the sides of her breasts, associated paraesthesia, and was experiencing both physical and psychological suffering due to her massive breasts. Her premorbid breast cup size was 36B, though, at the time of presentation, her breasts could no longer be supported by any brassiere. She was referred from a second level hospital clinic after initially being treated for breast cellulitis.
On clinical examination, her breasts were found to be massively enlarged bilaterally, erythematous, and painful, with the left more tender than the right. She also demonstrated thinning and hyperpigmentation of the skin, particularly in the lateral aspect. A breast ultrasound revealed multiple masses in both breasts, with the largest measuring 163 mm x 100 mm. An ultrasound guided core biopsy of the largest mass in the left breast demonstrated a fibroepithelial lesion with a mostly intracanalicular growth pattern and stromal morphology consistent with pseudo-angiomatous stromal hyperplasia (PASH). There were no morphological features suggestive of phyllodes tumour, and the biopsy was negative for in situ and invasive malignancy. At her first antenatal clinic visit, she was diagnosed with HIV and commenced on tenofovir/lamivudine/dolutegravir (TLD). She had one prior uncomplicated pregnancy.
The patient's biochemistry differed from the normal ranges evident in reproductive females, with prolactin (PRL), oestradiol (E2), progesterone (P4), and sex hormone binding globulin (SHBG) levels all above non-pregnancy ranges. The elevation of these hormones and binding protein is in keeping with the normal physiological changes of pregnancy and all within range for a patient at 16 weeks gestation (PRL: 82.0 ug/L [normal pre-pregnancy range: 4.8-23.3], SHBG: 320.9 nmol/l, E2: 7 869 pmol/L [2.47 ng/ml] and progesterone: 115.0 mmol/L). An isolated hypothyroxinaemia was also observed (thyroid stimulating hormone [TSH]: 2.39 mIU/L [0.27-4.20]; thyroxine [free T4]: 7.6 pmol/L [12.0-22.0] and tri-iodine thyronine [free T3]: 5.0 pmol/L [3.1-6.8]). She had no clinical features suggestive of thyroid dysfunction. The isolated hypothyroxinaemia was not evaluated further as treatment thereof is not currently recommended during pregnancy.1
Following the Tygerberg Hospital multidisciplinary endocrine meeting that included endocrine and breast surgeons, endocrinologists and pathologists, the decision was made to start the patient on a course of cabergoline at a conservative dosing of 0.25 mg twice weekly. Though her prolactin levels responded, measuring within the normal non-pregnancy range (23.5 ug/L) at three weeks, she clinically continued to exhibit a persistent increase in breast size.
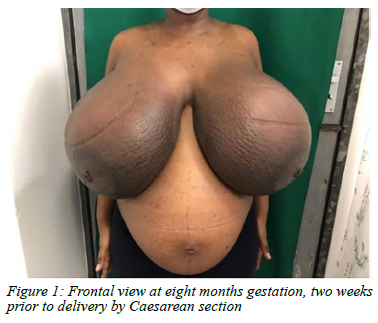
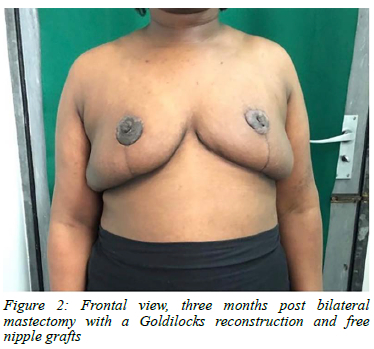
The patient underwent a caesarean section at 34 weeks gestation. The decision was based on threatening pressure necrosis of the skin, marked patient discomfort, and compression of the pregnant abdomen (Figure 1). The delivery was uncomplicated. One week following delivery the breasts began reducing in size. The breast involution continued but given the persistent extreme breast size at seven months post-delivery, a bilateral mastectomy with a Goldilocks reconstruction and free nipple grafts was performed. The weight of the excised tissue was 2435 g and 2195 g on the left and right, respectively. Histology confirmed the diagnosis of PASH bilaterally. Three months post-surgery, her breasts were well healed and she had full range of motion in her upper limbs. Her only complaints were the loss of nipple sensation and intermittent pruritus. Overall, the patient was satisfied with her cosmetic outcome (Figure 2).
Discussion
Gestational gigantomastia, also known as pregnancy related macromastia or hypertrophy, is a rare condition typified by excessive breast growth during pregnancy.23 No formal definition exists, though a surgical reduction of over 1500 g is often cited.2 The typical age range for pregnancy related gigantomastia is 20-30 years, with a reported prevalence ranging from 1 in 28 000 to 1 in 100 000.2 Gestational gigantomastia is rare, with less than sixty cases documented worldwide, and only one other in South Africa.4 The pathophysiology is poorly understood though a hormonal basis is suspected, with both excess hormone production and increased breast tissue sensitization hypothesised.256 The condition typically presents in the first trimester1 and may manifest after previous normal pregnancies.5 Patients present with rapid and persistent breast enlargement that may result in mastalgia, peau d'orange, cellulitis, decreased mobility, back pain, and loss of nipple sensation as well as psychosocial symptoms.2,3,6 Skin involvement may proceed to ulceration and localised infection.2,3 Medical therapy with dopamine agonists may be considered, however, surgery remains the mainstay of therapy in most cases.2 Recurrence with subsequent pregnancy is likely unless mastectomy has been performed, which lowers the rate thereof.2,6
PASH is a benign keloid-like proliferation of mammary stroma that histologically may simulate a vascular proliferating lesion. It is postulated to have a hormonal basis as it typically affects either premenopausal women or postmenopausal women on hormone therapy.7 PASH is found incidentally in up to 23% of biopsies evaluated for breast masses or abnormal mammographic findings.8 The tumorous form, however, is rare. Only around 200 such cases have been reported in the literature to date.8,9 PASH typically presents as a unilateral well circumscribed mass, or more rarely, as a bilateral diffuse mass.9 Our patient presented, anomalously, with bilateral nodular PASH, with the masses initially misdiagnosed as fibroadenomas on ultrasound. Aberrant stimulation of mammary myofibroblasts by P4 is proposed as potentially responsible for the abnormal stromal hyperplasia.7 PASH also occurs in the clinical setting of HIV infection. Impaired cellular immune response including decreased T-lymphocytic function may play a role in the pathogenesis of disease in these patients. However, the role of HIV in the pathogenesis of mammary PASH requires further investigation.10 Treatment of PASH can be individualised - incidental PASH typically requires no treatment, while tumorous PASH is excised with clear margins.7,9 Experience with the use of the selective oestrogen receptor modulator tamoxifen in the treatment of PASH is limited to singular case reports and thus not an established component of the definitive management.7,11
PRL, along with E2, P4 and growth hormone, is responsible for physiological breast changes in pregnancy to prepare for lactation postpartum. According to Dancey et al., prolactin hypersensitivity is a possible driving force behind gestational gigantomastia.2 Bromocriptine, a dopamine agonist able to suppress prolactin release from the anterior pituitary, is thus proposed in the literature as a non-surgical management option for gestational gigantomastia. Success reported with bromocriptine in the literature is variable.3 For this patient, cabergoline, a more potent dopamine agonist, was used. Although not routinely advocated for use in pregnancy, exposure to cabergoline in many normal pregnancies has not raised concern for teratogenic or other adverse foetal affectations.12,13 In this case, despite reducing blood PRL levels to normal pre-pregnancy values, breast growth continued. Other hormones implicated with gestational gigantomastia are E2 and P4,6 both expected to be significantly elevated during normal pregnancy, as evident in this patient.
Breast involution typically commences postpartum in patients with gestational gigantomastia, as was the case here.6 Surgical management includes both reduction mammoplasty and mastectomy options. Reduction mammoplasties have been performed successfully, though Swelstad et al. found that patients who underwent breast reductions had a 100% recurrence rate with subsequent pregnancies.6 Mastectomy has a lower risk of recurrence and the shorter operating time results in less blood loss.6 The Goldilocks procedure, first described by Richardson and Ma in 2012, presents a novel approach for reconstruction.14 In this case, a nipple sparing skin sparing mastectomy was performed. Enough skin was left for an inverted T-shaped reduction type pattern and the remaining skin (inferiorly) was de-epithelialised. This was then folded to create a breast. It is an excellent reconstructive technique for women with large breasts who want an immediate reconstruction of a smaller breast. We recommend two groups of surgeons operating simultaneously to reduce operating time.
In summary, we report a case of bilateral gestational gigantomastia complicated by tumoral PASH in a 27-year-old HIV-positive woman. The diagnosis of gigantomastia was suspected clinically and PASH was confirmed on histopathology. A multidisciplinary management approach was implemented and produced a satisfactory outcome for the patient. The medical management with cabergoline normalised her hyperprolactinaemia but failed to inhibit or slow breast growth. The Goldilocks procedure is an appropriate addition to the existing surgical options for gestational gigantomastia, especially as such cases often have dermal tissue excess.
Acknowledgements
We would like to acknowledge and thank Dr LI Martin for her contributions to the editing of this case report.
Conflict of interest
The authors report no conflict of interest.
Funding source
No funding was required.
Ethical approval
Ethical approval was obtained from the Stellenbosch University Research Ethics Committee (Ref: UC23/01/217). Patient consent for publication was obtained.
ORCID
FH Rabe https://orcid.org/0009-0008-4366-6589
M Comadie https://orcid.org/0000-0003-3092-4098
M Mahoko https://orcid.org/0000-0002-7850-8432
RC de Villiers https://orcid.org/0009-0000-6434-4847
J Edge https://orcid.org/0000-0003-3005-7254
REFERENCES
1. Alexander EK, Pearce EN, Brent GA, et al. 2017 guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27(3):315-89. https://doi.org/10.1089/thy.2016.0457. [ Links ]
2. Dancey A, Khan M, Dawson J, Peart F. Gigantomastia - a classification and review of the literature. J Plast Reconstr Aesthet Surg. 2008;61(5):493-502. https://doi.org/10.1016/j.bjps.2007.10.041. [ Links ]
3. Rezai S, Nakagawa JT, Tedesco J, et al. Gestational gigantomastia complicating pregnancy: A case report and review of the literature. Case Rep Obstet Gynecol. 2015;2015:1. https://doi.org/10.1155/2015/892369. [ Links ]
4. Rooi A, Botha A, Ndobe E, Nel M. Severe gestational gigantomastia - management challenges. S Afr J Surg. 2021;59(4). https://doi.org/10.17159/2078-5151/2021/v59n4a3547. [ Links ]
5. Zargar AH, Laway BA, Masoodi SR, et al. Gestational macromastia not responding to termination of pregnancy. Postgrad Med J. 1995;71(832):124-5. https://doi.org/10.1136/pgmj.71.832.124-b. [ Links ]
6. Swelstad MR, Swelstad BB, Rao VK, Gutowski KA. Management of gestational gigantomastia. Plast Reconstruct Surg. 2006;118(4):840-8. https://doi.org/10.1097/01.prs.0000232364.40958.47. [ Links ]
7. Virk RK, Khan A. Pseudo-angiomatous stromal hyperplasia: An overview. Arch Pathol Lab Med. 2010;134(7):1070-4. https://doi.org/10.5858/2008-0686-RS.1. [ Links ]
8. Ibrahim RE, Sciotto CG, Weidner N. Pseudo-angiomatous hyperplasia of mammary stroma. Some observations regarding its clinicopathologic spectrum. Cancer. 1989;63(6):1154-60. https://doi.org/10.1002/1097-0142(19890315)63:6<1154::AID-CNCR2820630619>3.0.CO;2-Q. [ Links ]
9. Krawczyk N, Fehm T, Ruckhaberle E, et al. Bilateral diffuse pseudo-angiomatous stromal hyperplasia (PASH) causing gigantomastia in a 33-year-old pregnant woman: Case report. Breast Care. 2016;11(5):356-8. https://doi.org/10.1159/000450867. [ Links ]
10. Larbcharoensub N, Wattanatranon D, Sanpaphant S, et al. Bilateral pseudo-angiomatous stromal hyperplasia in a human immunodeficiency viral-infected patient. Indian J Pathol Microbiol. 2015;58(3):356. https://doi.org/10.4103/0377-4929.162899. [ Links ]
11. Pruthi S, Reynolds C, Johnson RE, Gisvold JJ. Tamoxifen in the management of pseudo-angiomatous stromal hyperplasia. Breast J. 2001;7(6):434-9. https://doi.org/10.1046/j.1524-4741.2001.07611.x. [ Links ]
12. Molitch ME. Endocrinology in pregnancy: Management of the pregnant patient with a prolactinoma. Eur J Endocrinol. 2015;172(5). https://doi.org/10.1530/EJE-14-0848. [ Links ]
13. Stalldecker G, Mallea-Gil MS, Guitelman M, et al. Effects of cabergoline on pregnancy and embryo-foetal development: retrospective study on 103 pregnancies and a review of the literature. Pituitary. 2010;13(4):345-50. https://doi.org/10.1007/s11102-010-0243-6. [ Links ]
14. Richardson H, Ma G. The goldilocks mastectomy. Int J Surg. 2012;10(9):522-6. https://doi.org/10.1016/jijsu.2012.08.003. [ Links ]
 Correspondence:
Correspondence:
FH Rabe
Email: felix.rabe007@gmail.com














