Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Surgery
versión On-line ISSN 2078-5151
versión impresa ISSN 0038-2361
S. Afr. j. surg. vol.62 no.1 Cape Town 2024
http://dx.doi.org/10.36303/SAJS.00217
UPPER GASTRO-INTESTINAL SURGERY
Predicting survival in locally advanced gastric cancer using prognostic factors - neoadjuvant rectal score and downstaging depth score
S TamamI; S CulcuI; K ErözkanI; MS BenkI; C AziliI; E AltinsoyI; S ErsözII; AE UnalI
IDivision of Surgical Oncology, Department of Surgery, Ankara University Cebeci Hospital, Ankara University School of Medicine, Turkey
IIDepartment of Surgery, Ankara University Cebeci Hospital, Ankara University School of Medicine, Turkey
ABSTRACT
BACKGROUND: Clinical prediction models are needed to accurately predict the prognosis of patients with gastric cancer who have received neoadjuvant therapy and to determine the best treatment strategies. The aim of this study is to determine the role of two prognostic factors, the neoadjuvant rectal (NAR) score and the downstaging depth score (DDS), in predicting survival in patients with gastric cancer who received neoadjuvant therapy and underwent curative gastrectomy
METHODS: We reviewed the medical records of 129 patients who had been diagnosed with primary gastric cancer and underwent radical gastrectomy after receiving neoadjuvant therapy. We calculated the NAR score and DDS values for each patient and conducted a survival analysis to assess the accuracy of these prognostic factors in predicting overall survival
RESULTS: The median overall survival time of the patients was found to be 29 months. Patients with low NAR scores and high DDS had significantly longer overall survival. Univariate analyses based on clinical and laboratory characteristics showed that gender, surgery type, resection type, neural invasion, grade, adjuvant radiotherapy, lymphocyte level, carcinoembryonic antigen (CEA) level, NAR score, and DDS were associated with survival. Moreover, multivariate analyses showed that lymphocyte level, DDS, and NAR score were independent prognostic factors
CONCLUSIONS: In summary, our research indicates that NAR score and DDS may serve as useful prognostic markers for predicting overall survival in patients with locally advanced gastric cancer who receive neoadjuvant chemotherapy followed by curative surgery. Patients with high DDS and low NAR scores were found to have better prognoses
Keywords: locally advanced gastric cancer, neoadjuvant rectal score, downstaging depth score, gastric cancer, neoadjuvant therapy
Introduction
Gastric cancer is often diagnosed in advanced stages and is one of the leading causes of cancer-related deaths worldwide.1-3 Despite advancements in surgical techniques, chemotherapy, and radiation therapy, the five-year survival rate for advanced gastric cancer is only around 5-20%, with a median overall survival of less than one year.4 Even after radical resection, many patients experience recurrence or metastasis within five years. It is important to develop a treatment plan that corresponds with the patient's expected lifespan to minimise the risk of recurrence and metastasis.
Neoadjuvant therapy has been shown to benefit localised disease in phase III studies,5,6 and multimodality treatment has become a standard approach.6,7 However, even patients with the same stage of disease and receiving similar treatments may have different prognoses. Therefore, accurate prediction of the prognosis of patients with gastric cancer and the determination of the optimal treatment strategy requires the use of clinical prediction models and further research.
Clinical prediction models are increasingly being used to predict prognosis and survival in cancer patients. The ypTNM staging system and tumour regression grade defined by the American Joint Committee on Cancer (AJCC) are markers that are associated with prognosis and survival and help determine appropriate treatment. However, these markers may have some limitations in predicting prognosis accurately as they do not consider pre-neoadjuvant therapy staging. While the benefits of neoadjuvant chemotherapy in gastric cancer have been demonstrated, early indicators of disease progression in patients who receive neoadjuvant chemotherapy are still lacking.
The downstaging depth score (DDS) and neoadjuvant rectal (NAR) score have recently been identified as predictors of survival in patients with neoadjuvant-treated rectal cancer.8-10 The aim of this study is to determine the role of DDS and NAR score in predicting survival in patients with gastric cancer who have received neoadjuvant therapy followed by curative gastrectomy.
Methods
Patients and data
After the approval (date: 30.12.2022, No: Í11-686-22) was granted by our ethics committee, a retrospective analysis was made on the Ankara University patient database from 2007 to 2019.
We analysed 129 patients who underwent radical gastrectomy after neoadjuvant therapy for advanced local gastric adenocarcinoma. Patients under the age of 18, with severe systemic disease - chronic renal failure, chronic liver failure, severe pulmonary disease, etc. - synchronous tumour, pathology other than adenocarcinoma or signet ring cell adenocarcinoma, and who underwent emergency or palliative surgery were excluded from the study. A total of 74 patients were found to be eligible for the study. The patients' age at diagnosis, location of primary tumour, type of surgery, adjuvant/neoadjuvant treatment protocol, pathological results, clinical-pathological tumour stage and lymph node involvement, preoperative laboratory results, levels of carcinoembryonic antigen (CEA) and cancer antigen 19-9 (CA 19-9), presence of vascular and neural invasion, and number of removed and metastatic lymph nodes were obtained from the hospital database.
The patients were staged according to the 8th edition of the AJCC tumour/node/metastasis (TNM) clinical and pathological staging system.11,12 Thoraco-abdominopelvic computed tomography (CT) was used as standard for staging. Other staging methods such as gastroscopy, endoscopic ultrasound or abdominal magnetic resonance imaging (MRI) were also used in case of clinical suspicion. The neoadjuvant treatment protocols were divided into three groups - modified DCF (docetaxel 60 mg/m2, cisplatin 60 mg/m2, and 5-fluorouracil 600 mg/m2/day (5 days) infusion, every 21 days), FLOT (5-fluorouracil 2600 mg/m2/day 24-hour infusion, leucovorin 200 mg/m2, oxaliplatin 85 mg/ m2, docetaxel 50 mg/m2 on day 1, every 14 days), and other chemotherapy protocols (ECF - epirubicin, cisplatin, 5-fluorouracil, ECX - epirubicin, cisplatin, capecitabine). The presence of adjuvant chemotherapy and radiotherapy was recorded. All included patients underwent D2 dissection independent of the type of surgery.
The patients' NAR score and DDS were calculated. The DDS was recorded before and after the operation. The relationship between the NAR score and DDS with prognosis was analysed. The area under the receiver operator characteristics (ROC) curves was calculated. The accuracy of the NAR score and DDS in predicting overall survival was evaluated. Preoperative haemoglobin, albumin, leukocyte, lymphocyte, platelet, and neutrophil values were also classified as low, normal, and high based on the laboratory reference range. Tumour markers were classified as normal and high based on the laboratory reference range. The neutrophil-lymphocyte ratio (NLR), an inflammatory marker, was calculated using the formula neutrophil count/ lymphocyte count.
NAR score
The NAR score is designed to be a representative endpoint in clinical trials that is sensitive to factors affected by neoadjuvant therapy. It uses universal clinicopathological factors in clinical trials, which makes it cost-effective and time-efficient.13 The NAR score is calculated using the following formula - [5 ypN - 3 (cT - ypT) + 12]2 /9.61. The cT (0, 1, 2, 3, 4) in the NAR score denotes clinical stage, while pT (0, 1, 2, 3, 4) and pN (0, 1, 2, 3) represent pathological T and N stages, respectively.
DDS
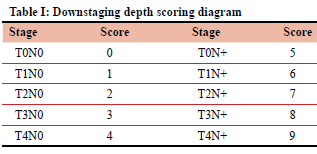
DDS is a response evaluation method that uses the TNM staging system. T stages 0-4 and N stage 0 are scored 0-4 points, while T stages 0-4 and N stage + are scored 5-9 points (Table I). The preoperative score is evaluated based on the clinical stage, while the postoperative score is based on pathological findings. The final score is calculated by subtracting the post-treatment score from the pre-treatment score (final DDS = pre-treatment score - post-treatment score).10
Statistical analysis
The statistical analysis was performed using the Statistical Package for Social Sciences (SPSS version 25, IBM Corp. NY, USA) computer software package for Windows. Categorical variables were presented as counts and percentages, while continuous variables were presented as mean ± standard deviation and median (interquartile range, 25th-75th percentile) in the descriptive statistics section. The chi-square test was used for comparison analysis of categorical variables between independent groups. Normal distribution of continuous variables was evaluated using coefficient of variation, histogram graphs, and the Kolmogorov-Smirnov test. For parametric cases where the data was normally distributed, t-tests were used for the analysis between two independent groups, while nonparametric cases where the data was not normally distributed, the Mann-Whitney U test was used. Analysis of three or more groups was performed using ANOVA for normally distributed data and Kruskal-Wallis test (Bonferroni-corrected Mann-Whitney U test) for non-normally distributed data. Visual (histogram and probability graphs) and analytical methods (Kolmogorov-Smirnov/Shapiro-Wilk tests) were used to investigate the normality of numerical variables. When the variables were normally distributed, correlation coefficients and statistical significance were calculated using the Pearson test, and when not, the Spearman test was used.
ROC analysis was used to determine the optimal cutoff values for NAR and DDS. Optimal cutoff values were calculated by analysing the area under the curve using the Youden index.
Kaplan-Meier test was used for survival analysis, and the results were analysed using the Log-rank test. The overall survival (OS) duration of patients was defined as the time elapsed between the diagnosis date and the date of death for any reason. Univariate and multivariate analyses for OS were performed using the Cox regression model. Variables that were found to be significant in the univariate analysis were included in the multivariate regression analysis model. Prognostic factors affecting survival were examined in the multivariate analysis. Cases where the type 1 error level was below 5% were considered statistically significant. In this study, a general level of statistical significance of p < 0.05 was accepted.
Results
Demographics and tumour characteristics
The study group consisted mostly of males (60.8%) with a mean age of 59 ± 10.1 years. The most frequent tumour location was the antrum (51.4%). The most frequent type of surgery was total gastrectomy (n = 44, 59.5%), and subtotal gastrectomy was performed in 31.5% of the patients. An R0 resection was achieved in all patients. Preoperative clinical (radiological) lymph node positivity was found in 82.4% (n = 61) of the patients, but this rate decreased to 73% (n = 54) following postoperative pathological review. Clinical T4 tumour was detected in 71.6% of patients (n = 53), but this decreased to 62.2% (n = 46) after pathological staging. All patients received neoadjuvant chemotherapy based on inclusion criteria, with the most frequently used regimen being modified DCF (74.3%). The second most frequent regimen was FLOT (17.6%). Adjuvant chemotherapy was given to the majority of patients (68.9%), and 23% of patients received adjuvant radiotherapy.
Pathological analysis showed that 62.2% of tumours were high-grade or poorly differentiated adenocarcinomas. The preoperative and postoperative diagnoses of all patients were adenocarcinoma. Of the preoperative small biopsy specimens, 93.2% were adenocarcinoma subtypes other than signet ring cell adenocarcinoma, while 6.8% were signet ring cell adenocarcinoma. After resection and complete pathologic evaluation with additional specialised stains such as cytokeratin immunohistochemistry, 70.3% were adenocarcinoma subtypes other than signet ring cell adenocarcinoma and 29.7% were signet ring cell adenocarcinoma. Vascular and neural invasion were found in 37.8% and 43.2% of patients, respectively. The median number of removed lymph nodes was 22 (range 16-46), with a median of 3 (range 0-30) metastatic lymph nodes. The median lymph node ratio was 0.15 (range 0-0.95). The median NAR score was 45.99, and the median final DDS was 0 (range -6 to 8). Patients were divided into two groups based on final DDS (group 1: < 0; group 2: > 0).
Results of ROC analysis
To assess the prognostic value of the NAR score in predicting survival, we conducted a ROC analysis. The area under the curve (AUC), which represents the accuracy of the test, was 0.843 with a 95% confidence interval (CI) of 0.746-0.941 and a _p-value of less than 0.001. The optimal cutoff value for the NAR score was determined to be 33.81, with a sensitivity of 78.7% and a specificity of 70.4%. The Youden index, a measure of the effectiveness of the cutoff point, was calculated to be 0.491. Based on the NAR score, patients were grouped into high-risk (> 33.81) and low-risk (< 33.81) categories. Clinical and laboratory characteristics of the patients were compared between these groups (Table II).
Patients were also grouped based on their median DDS as high-risk (DDS < 0) and low-risk (DDS > 0). The clinical and laboratory characteristics of patients based on the DDS were presented in Table III.
Survival analysis
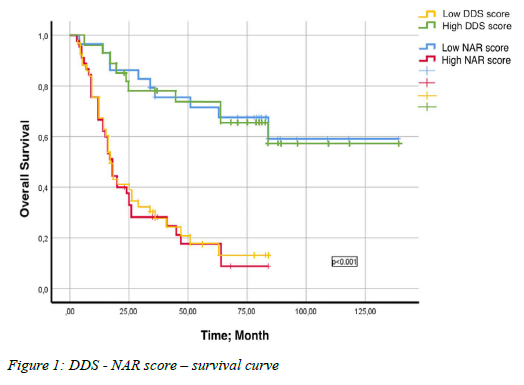
The median overall survival time of patients during the 27.5 months (3-139 months) follow-up period was found to be 29 months (95% CI; 10.9-47.0). Survival analysis based on NAR score and DDS showed that patients with low NAR score and high DDS had significantly longer overall survival (Table IV, Figure 1). In addition, significant survival differences were obtained among variables such as gender, surgical type, vascular invasion, neural invasion, grade, and neoadjuvant chemotherapy type (Table IV). No survival difference was observed based on tumour location (p > 0.05).
Univariate and multivariate analyses
Univariate analysis revealed that gender, surgery, resection type, neural invasion, grade, adjuvant radiotherapy, lymphocyte level, CEA level, NAR score, DDS, and NLR variables affected survival (Table V). Variables that yielded significant results in univariate analysis were evaluated in multivariate analysis to determine whether they were independent prognostic factors. Multivariate analysis showed that lymphocyte level, DDS, and NAR score were independent prognostic factors. The analysis revealed that a lymphocyte level between 1000-4000 reduced the risk of death by 0.29-fold (p = 0.008), high DDS reduced the risk of death by 0.16-fold (p < 0.001), and high NAR score increased the risk of death by 4.3-fold < 0.001) (Table V).
Discussion
While neoadjuvant therapy is promising in the treatment of locally advanced gastric cancer patients, there are still few prognostic indicators that can guide the strategy to be followed after neoadjuvant therapy. With the increasing benefits of the neoadjuvant treatment approach, predicting the disease course based on the tumour response to chemotherapy in this population has become important. Clinical-pathological factors and clinical prediction models have been discussed in many studies and are increasingly being used to predict prognosis and survival in cancer patients. The histological response (tumour regression grade) after neoadjuvant therapy and the ypTNM staging system defined by AJCC for selecting appropriate treatment are generally accepted as markers associated with prognosis and survival.14-18 However, these markers may have some limitations in accurately predicting prognosis as they do not take into account the pre-neoadjuvant therapy staging.
Therefore, we planned this study to evaluate the role of pre-neoadjuvant therapy staging and DDS and NAR score in predicting survival in patients with gastric cancer who received neoadjuvant therapy followed by curative gastrectomy.
While previously validated in rectal cancer,8-10 we demonstrated that DDS and NAR scores may have prognostic value in patients who received radical gastrectomy after neoadjuvant chemotherapy. According to our results, high DDS and low NAR scores in locally advanced gastric cancer patients, who underwent curative surgery after neoadjuvant chemotherapy, indicate better survival. Similar results were obtained in the multivariate analysis.
In clinical trials, efforts to discover a surrogate endpoint have led to the development of a new scoring system called the NAR score for patients with rectal cancer who receive neoadjuvant therapy.13 Recent studies have shown that the NAR score also has prognostic value in rectal cancer patients receiving neoadjuvant chemoradiotherapy.19-21 According to these studies, the NAR score can be useful in selecting patients who could benefit from adjuvant chemotherapy and in predicting survival. We suggest that the prognostic value of the NAR score previously developed for rectal cancer, can be extrapolated to gastric cancer as there are significant similarities between the staging systems for these two types of cancer. Primary tumour staging is solely based on tumour invasion depth both in gastric and rectal cancer. Also, the number of positive lymph nodes for nodal (N) staging has only minor differences between gastric and rectal cancer.
Looking at the literature, patients with a NAR score above 16 in rectal cancer are classified as being in the high-risk group.13 We determined the threshold for the NAR score in our gastric cancer cohort to be 33.81, and similarly noted that lower NAR scores were consistent with better prognosis, as in the rectal cancer literature.9,13
DDS is a new prognostic evaluation method that can predict prognosis by considering the T and N stages before and after treatment. In patients with locally advanced rectal cancer who receive neoadjuvant therapy, higher DDS values indicate a better response to treatment and a better prognosis. Ning Li et al. showed that the prognostic value of DDS in rectal cancer is better than pathological complete response (pCR).10 In this study, patients with DDS less than 4 were classified as being in the high-risk group. In the current study, we set the threshold value for DDS at 0 and we observed that higher DDS scores were a good prognostic indicator.
According to our knowledge, this is the first study to evaluate the prognostic value of both the NAR score and DDS in predicting prognosis of gastric cancer. However, there are some limitations to our study that may affect the results. It is a retrospective, single-center study with a small sample size. There are several clinicopathological factors such as tumour size and adenocarcinoma histological subtypes - tubular, mucinous, mixed, etc. - that may be related to prognosis but were not included in this study. Since our study aimed to develop a scoring system, we wanted to homogenise the study. In addition, these clinicopathologic factors were not mentioned in some of the postoperative pathology reports. Therefore, we did not include these factors in the study. Additionally, although the treatment modality was similar for most patients, many included in the study did not receive the current standard chemotherapy regimen of FLOT.
This study demonstrated the potential prognostic value of NAR score and DDS in gastric cancer in cohort subsets, although most patients did not receive the current standard chemotherapy regimen (FLOT). Our study serves as a guide for future studies with larger populations. In order to validate the DDS and NAR score in gastric cancer patients and to introduce it into current use, our studies are continuing in patient groups who have received current treatment.
MRI is generally used when calculating the NAR score in rectal cancer. However MRI has played a relatively limited role in the locoregional staging of gastric cancer. This is primarily due to limited accessibility and long examination times leading to respiration and peristaltic motion artifacts. Therefore, CT is generally used more frequently in gastric cancer staging. We also used CT for staging in all patients included in our study. We believe that examining more homogeneous and broader patient groups that include gastric cancer will confirm the prognostic value of NAR score and DDS.
In patients with locally advanced gastric cancer who underwent curative surgery after neoadjuvant chemotherapy, NAR score and DDS may serve as predictive markers for survival. High DDS and low NAR score are indicative of a good prognosis. These findings may have important clinical implications for developing more effective treatment strategies and improving the outcomes of patients with gastric cancer. However, to determine which score is more valuable, more comprehensive randomised studies are needed.
Acknowledgments
The authors express their gratitude to the patient who made this work possible, as well as the professionals and researchers who participated in this study.
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
Ethical committee approval was obtained from the Insan Arastirmalari Etik Kurulu Karari Ethics Committee (Ref: 111-686-22). Informed consent was obtained from all patients.
ORCID
S Tamam https://orcid.org/0000-0002-2924-1874
S Culcu https://orcid.org/0000-0002-1136-1771
K Erozkan https://orcid.org/0000-0003-2193-9984
MS Benk https://orcid.org/0000-0001-9022-7353
C Azili https://orcid.org/0000-0003-3661-2052
E Altinsoy https://orcid.org/0000-0002-0486-1284
S Ersöz https://orcid.org/0000-0003-1824-8453
AE Unal https://orcid.org/0000-0002-2757-4034
REFERENCES
1. Global Burden of Disease Cancer Collaboration. The Global Burden of Cancer 2013 2015;1(5):690. https://doi.org/10.1001/jamaoncol.2015.0735. [ Links ]
2. Badgwell B. Multimodality therapy of localized gastric adenocarcinoma. J Natl Compr Canc Netw. 2016;14(10):1321-7. https://doi.org/10.6004/jnccn.2016.0139. [ Links ]
3. Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941-53. https://doi.org/10.1002/ijc.31937. [ Links ]
4. Powell AGMT, Parkinson D, Patel N, et al. Prognostic significance of serum inflammatory markers in gastric cancer. J Gastrointest Surg. 2018;22(4):595-605. https://doi.org/10.1007/s11605-017-3597-5. [ Links ]
5. Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: An FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29(13):1715-21. https://doi.org/10.1200/JCO.2010.33.0597. [ Links ]
6. Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11-20. https://doi.org/10.1056/NEJMoa055531. [ Links ]
7. Ajani JA, Winter K, Okawara GS, et al. Phase II trial of preoperative chemoradiation in patients with localised gastric adenocarcinoma (RTOG 9904): Quality of combined modality therapy and pathologic response. J Clin Oncol. 2006;24(24):3953-8. https://doi.org/10.1200/JCO.2006.06.4840. [ Links ]
8. Roselló S, Frasson M, García-Granero E, et al. Integrating downstaging in the risk assessment of patients with locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy: Validation of valentini's nomograms and the neoadjuvant rectal score. Clin Colorectal Cancer. 2018;17(2):104-12.e2. https://doi.org/10.1016/j.clcc.2017.10.014. [ Links ]
9. Sun Y, Zhang Y, Wu X, et al. Prognostic significance of neoadjuvant rectal score in locally advanced rectal cancer after neoadjuvant chemoradiotherapy and construction of a prediction model. J Surg Oncol. 2018;117(4):737-44. https://doi.org/10.1002/jso.24907. [ Links ]
10. Li N, Jin J, Yu J, et al. Downstaging depth score to predict outcomes in locally advanced rectal cancer achieving ypI stage after neoadjuvant chemo-radiotherapy versus de novo stage pI cohort: A propensity score-matched analysis. Chin J Cancer Res. 2018;30(3):373-81. https://doi.org/10.21147/j.issn.1000-9604.2018.03.09. [ Links ]
11. In H, Solsky I, Palis B, et al. Validation of the 8th edition of the AJCC TNM staging system for gastric cancer using the national cancer database. Ann Surg Oncol. 2017;24(12):3683-91. https://doi.org/10.1245/s10434-017-6078-x. [ Links ]
12. Graziosi L, Marino E, Donini A. Survival comparison in gastric cancer patients between 7th and 8th edition of the AJCC TNM staging system: the first western single center experience. Eur J Surg Oncol. 2019;45(6):1105-08. https://doi.org/10.1016/j.ejso.2018.12.010. [ Links ]
13. George TJ Jr, Allegra CJ, Yothers G. Neoadjuvant rectal (NAR) score: A new surrogate endpoint in rectal cancer clinical trials. Curr Colorectal Cancer Rep. 2015;11(5):275-80. https://doi.org/10.1007/s11888-015-0285-2. [ Links ]
14. Wang X, Li X, Zhou N, et al. Graded histologic response after neoadjuvant chemotherapy is an optimal criterion for treatment change in patients with locally advanced gastric cancer. Ann Transl Med. 2019;7(20):546. https://doi.org/10.21037/atm.2019.09.82. [ Links ]
15. Tang X, He Q, Qu H, et al. Post-therapy pathologic tumour volume predicts survival in gastric cancer patients who underwent neoadjuvant chemotherapy and gastrectomy. BMC Cancer. 2019;19(1):797. https://doi.org/10.1186/s12885-019-6012-7. [ Links ]
16. Rawicz-Pruszynski K, Cisel B, Mlak R, et al. The role of the lymph node ratio in advanced gastric cancer after neoadjuvant chemotherapy. Cancers (Basel). 2019;11(12):1914. https://doi.org/10.3390/cancers11121914. [ Links ]
17. Pereira MA, Ramos MF, Dias AR, et al. Lymph node regression after neoadjuvant chemotherapy: A predictor of survival in gastric cancer. J Surg Oncol. 2020;121(5):795-803. https://doi.org/10.1002/jso.25785. [ Links ]
18. Sanchez de Molina ML, Diaz Del Arco C, Vorwald P, et al. Histopathological factors predicting response to neoadjuvant therapy in gastric carcinoma. Clin Transl Oncol. 2018;20(2):253-7. https://doi.org/10.1007/s12094-017-1707-1. [ Links ]
19. Mukai T, Uehara K, Aiba T, et al. Importance of the neoadjuvant rectal (NAR) score to the outcome of neoadjuvant chemotherapy alone for locally advanced rectal cancer. Surg Today. 2020;50(8):912-9. https://doi.org/10.1007/s00595-020-01964-1. [ Links ]
20. Maeda K, Shibutani M, Tachimori A, et al. Prognostic significance of neoadjuvant rectal score and indication for postoperative adjuvant therapy in rectal cancer patients after neoadjuvant chemoradiotherapy. In Vivo. 2020;34(1):283-9. https://doi.org/10.21873/invivo.11772. [ Links ]
21. Fokas E, Fietkau R, Hartmann A, et al. Neoadjuvant rectal score as individual-level surrogate for disease-free survival in rectal cancer in the CAO/ARO/AIO-04 randomised phase III trial. Ann Oncol. 2018;29(7):1521-7. https://doi.org/10.1093/annonc/mdy143. [ Links ]
 Correspondence:
Correspondence:
S Tamam
Email: selimtamam@hotmail.com














