Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Surgery
versión On-line ISSN 2078-5151
versión impresa ISSN 0038-2361
S. Afr. j. surg. vol.62 no.1 Cape Town 2024
http://dx.doi.org/10.36303/SAJS.00204
PAEDIATRIC SURGERY
Open abdominal wall defects and open spina bifida at a regional hospital in northern KwaZulu-Natal – bellwether conditions for neonatal surgery capacity
R VoslooI, II; G WyerII; L NaidooI, III; B EnickerIV, V; AG MaharajVI, VII; NC KapongoI, II
IDepartment of Paediatrics and Child Health, College of Health Sciences, School of Clinical Medicine, University of KwaZulu-Natal, South Africa
IIDepartment of Paediatrics, Queen Nandi Regional Hospital, South Africa
IIIDepartment of Paediatrics (Neonatal ICU), Inkosi Albert Luthuli Central Hospital, South Africa
IVDepartment of Neurosurgery, Inkosi Albert Luthuli Central Hospital, South Africa
VNelson R Mandela School of Medicine, University of KwaZulu-Natal, South Africa
VIDepartment of Paediatric Surgery, Inkosi Albert Luthuli Central Hospital, South Africa
VIIDepartment of Paediatric Surgery, College of Health Sciences, School of Clinical Medicine, University of KwaZulu-Natal, South Africa
ABSTRACT
BACKGROUND: Abdominal wall defects (AWDs), such as gastroschisis and omphalocele, and neural tube defects (NTDs) such as open spina bifida (SB) are common congenital anomalies. These anomalies are considered a leading cause of neonatal mortality and have been advocated as bellwether conditions to measure access to surgical care
METHODS: Newborns with open SB or AWD presenting to the nursery at Queen Nandi Regional Hospital over four years (2018-2021) were retrospectively identified. Clinical and electronic database records were reviewed to determine if transfers to definitive tertiary care occurred timeously. Reasons for delays and associated morbidity and/or mortality were investigated
RESULTS: Sixty-five patients were identified and two were excluded due to unavailable or incomplete records. It took a median of 8 days (IQR 2-18 days) to reach tertiary care, with SB cases waiting significantly longer (median 16 days, IQR 8-25 days) (p = 0.000). Lack of tertiary service capacity was the main reason for delays. The COVID-19 pandemic did not affect time intervals (p = 0.676). Complications were common and overall mortality at our facility was high (n = 11/63, 17.46%
CONCLUSION: Newborns with open SB or AWDs experience marked delays in reaching definitive care. This is more pronounced for cases of SB and was not influenced by the pandemic. Lack of tertiary service capacity (including bed availability, limited staff, and theatre time) is the most important limiting factor
Keywords: neonatal surgery, open spina bifida, open abdominal wall defect, access to care
Introduction
Almost 10% of all newborn deaths in sub-Saharan Africa are due to congenital anomalies.1 These anomalies include defects in structure, function or metabolism leading to disability and about half are surgical in nature.1,2 Accurate data are scarce in South Africa.3 By improving access to care, this high death rate could be decreased and future disease burden potentially minimised.13
Two of the commonest congenital anomalies requiring urgent surgical intervention include open abdominal wall defects (AWDs) and neural tube defects (NTDs).4 Poor outcomes are related to delays in reaching the appropriate level of care. AWDs include ectopia cordis, bladder exstrophy, gastroschisis and omphalocele.5 Gastroschisis has been suggested as a bellwether condition to assess both access to neonatal surgical services and outcomes.6 NTDs include anencephaly, encephalocele and spina bifida (SB) and the most common overt form of SB is myelomeningocele (MMC).7 Similarly, MMC closure has also been advocated as a bellwether procedure for paediatric neurosurgical services.8 Limited resources in KwaZulu-Natal lead to delays and many complications.9 The aim of this study was to determine whether newborns with open AWD or SB in northern KwaZulu-Natal are transferred for urgent surgical intervention at the tertiary hospital timeously (i.e. within 24 hours of birth) and to investigate the reasons for delays, associated complications and mortality.
Methods
Design
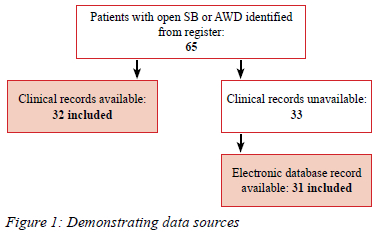
This was an observational retrospective study. Patients with either open SB or AWD admitted to the nursery from January 2018 to December 2021 were identified. Clinical records were obtained and supplemented by the local electronic database (Figure 1).
Setting
Queen Nandi Regional Hospital (QNRH) is a maternal, child and women's health facility in Empangeni, KwaZulu-Natal. Three of the 11 health districts in the province refer to the hospital, which provides mostly level 2 and some level 3 care. These districts include King Cetshwayo (mixed urban rural), uMkhanyakude (rural) and Zululand (rural) with an estimated population of 2.5 million (almost V of the provincial population).10
The 92-bed nursery maintains 16 intensive care (IC) and 40 high care (HC) beds. Invasive and non-invasive ventilation as well as inotropic support are provided in the neonatal intensive care unit (NICU) while open lesion care and total parenteral nutrition is delivered in the HC area if NICU level treatment is not required.
There are no paediatric surgery or neurosurgical services available at the hospital or the local tertiary hospital (Ngwelezana Hospital). These services are located at Inkosi Albert Luthuli Central Hospital (IALCH) in Durban as well as Greys Tertiary Hospital in Pietermaritzburg.
Data analysis
Data were collected using the REDCap electronic data capture tool hosted at the University of KwaZulu-Natal and analysed using STATA 14 (StataCorp, USA). Most continuous data were skewed and described using the median and interquartile range (IQR). In select data where gaussian distribution was seen, the mean and standard deviation (SD) were also described. Categorical data were reported as percentages. These data were analysed using the x2 test for significance. Continuous variables were tested using the Bonferroni analysis of variance for normally distributed values, and the Kruskal-Wallis test for nonparametric data. Significance was defined as p = < 0.05.
Results
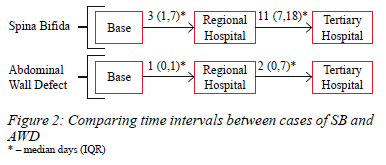
Sixty-five patients were identified, and data were available for 63. More than half were male (n = 35/63, 55.56%) (Table I). It took a median of 8 days (IQR 2-18 days) to reach tertiary care, with SB cases waiting significantly longer (p = 0.000) (Figure 2). The reasons for delays from QNRH to the tertiary hospital were available in 17 patients. Lack of tertiary beds, compounded by theatre time and staff availability, was the main reason identified. In 4 cases (n = 4/17, 25.53%) during the pandemic, SARS CoV-2 PCR results were also awaited. Complications were common (Table II) and almost a fifth of patients died at our facility (n = 11/63, 17.46%).
The majority were born via normal vaginal delivery (n = 36/63, 57.14%) (p = 0.492, by diagnostic group) (Table I). Most were born at term gestational age, with no significant difference observed between groups (p = 0.492). Weight was available for all the patients; however, length was only available for 27 (SB n = 11/27, gastroschisis n = 11/27, omphalocele n = 5/27) and head circumference for only 26 patients (SB n = 11/26, gastroschisis n = 10/26, omphalocele n = 5/26) (Table III). Anthropometry was similar across the study groups; however, some cases of macrocephaly, the largest measuring 47 cm, were observed in SB. The mean weight observed was 2465 g (742 g SD) and the mean length was 48 cm (3 cm SD).
The median maternal age was 24 years (IQR 20-31 years). There was a significant difference between maternal age in SB cases (median 31, IQR 24-34 years) compared to AWD (median 21, IQR 19-27 years) (p = 0.000). Only one (n = 1/63, 1.59%) mother did not attend antenatal care. However, most (n = 60/63, 95.24%) did not have a documented antenatal ultrasound scan. Of those with available scan results, one baby with SB had a normal late antenatal scan, another with AWD was part of a twin pregnancy with no abnormalities demonstrated. Only one baby with an omphalocele had an abnormal scan noting herniation of abdominal contents.
Gastroschisis made up most AWD cases (n = 24/35, 68.57%) with the remainder being cases of omphalocele.
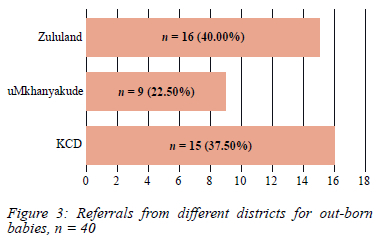
Twenty-three of the patients (n = 23/63, 36.51%) were born at QNRH (7 SB, 16 AWD) and the rest (n = 40/63, 63.49%) were out-born (21 SB, 19 AWD) (Figure 3).
Data on the time taken to reach tertiary care were available for 39 patients, while the time to reach our facility from the primary facility was available for 38 patients. Missing data were present in two patients who were transferred back to the base hospital to await a tertiary bed, and 11 others where records were unavailable or incomplete. The transfer time from the primary facility to QNRH was shorter in cases of AWD (SB n = 21/38, AWD n = 17/38) (p = 0.011) (Figure 2). This shorter time-to-transfer for AWD was also evident from regional to tertiary care (SB n = 17/39, AWD n = 22/39) (p = 0.003). All the tertiary referrals were transferred to IALCH, except for one patient with gastroschisis who was transferred to Greys Tertiary Hospital. The overall time interval from the primary facility to the tertiary hospital was longer for cases of SB (SB: median 16 days, IQR 8-25 days, n = 17/39; AWD: median 3 days, IQR 1-8 days, n = 22/39) (p = 0.000). Data comparing gastroschisis and omphalocele cases were available in 22 cases and there was no difference in the time to reach the tertiary facility (p = 0.192).
The advent of the COVID-19 pandemic did not influence the overall time to reach tertiary care (pre-COVID-19: median 8 days, IQR 3-18 days, n = 15/39; during-COVID-19: median 7 days, IQR 2-17 days, n = 24/39) (p = 0.676). No difference was observed comparing SB and AWD cases before and during the pandemic (SB p = 0.676, AWD p = 0.688).
Data on comorbidities and complications were only available where clinical records were retrieved. Most experienced complications while awaiting transfer (n = 22/32, 68.75%) (Table II). Hydrocephalus, diagnosed on bedside ultrasound, was present in almost half of SB cases (n = 7/15, 46.67%). Clubfoot deformities were also relatively common (n = 4/15, 26.67%). Dysmorphisms were noted in both groups (n = 5/32, 15.63%) including one patient with features of Waardenburg syndrome Type 1 (SB), one with features of Beckwith-Wiedemann syndrome (omphalocele), and three cases of SB with nonspecific dysmorphic features. Congenital heart disease was uncommon with only one combined ventricular septal defect and patent ductus arteriosus (SB), and one atriol septal defect (AWD) on informal bedside echocardiography.
Of the 11 patients who died at QNRH, six had AWD (n = 6/35, 17.14%), with the majority being gastroschisis (n = 5/6, 83.33%), while five had SB (n = 5/28, 17.86%). Most were classified as early neonatal deaths (median 2 days, IQR 3-9) and there was no difference in this time interval between the two categories (p = 0.235). Babies born at QNRH had a significantly higher chance of dying (p = 0.040) with seven inborn mortalities and four out-born mortalities. Clinical information on complications, comorbidities and the events surrounding death was only available for three patients. One had an omphalocele and presented with pneumonia and respiratory failure requiring mechanical ventilation. The baby developed shock and died on day one of life. The second had an open SB with hydrocephalus and demised on day eight of life. This patient had a leaking lesion, developed seizures, and died while awaiting transfer. Lack of tertiary bed access was the main reason for the delay. Both cases were born at QNRH. The last case was referred from Zululand district and had SB with hydrocephalus as well as pneumonia. The patient was too unstable for tertiary discussion early in life and developed complications including late infection, meningitis, seizures, intraventricular haemorrhage, and electrolyte derangements. He was later accepted for tertiary transfer but died on day 48 of life while awaiting transfer.
Discussion
Neonates requiring urgent definitive surgical care experience marked delays in reaching the tertiary service in our setting. This is more pronounced for cases of SB compared to AWD with tertiary service capacity, such as bed availability, limited staff, and theatre time, being the most important factor resulting in delays. This resulted in many complications and a high mortality rate, although somewhat lower than other contemporary reports. Reporting on cases of gastroschisis at IALCH, Sekabira et al. reported an in-hospital mortality of more than 40% for cases of gastroschisis from 2002 to 2007, while in a larger recent cohort including other centres in South Africa and other middle income countries, gastroschisis mortality was around 30% and omphalocele case mortality was lower at around 20%.1112 These studies did, however, include outcomes following surgery as well. Ikol et al. reported a higher mortality in neonates still awaiting surgery in a comparable setting (25%).13 A lower mortality was seen in a recent review of MMC management at IALCH (9%), however, complications were common.9 The need for further expansion of neonatal surgical services in our setting is evident.
Early closure of AWDs is preferred to limit infections, vascular compromise and mechanical injury from bowel exposure, while very large and very small omphaloceles can be managed conservatively.14 Cases of gastroschisis are admitted to the tertiary NICU and operative intervention occurs shortly after arrival as an emergency. Since delaying transfer in gastroschisis results in more complications and mortality when compared to omphalocele, it was expected that gastroschisis cases would reach tertiary services quicker. However, no difference was noted. Limited data most likely confounded this observation.
Open SB should be repaired within 24 hours after delivery as longer delays are associated with wound sepsis and other complications.9 SB cases are admitted to tertiary HC beds and are operated once theatre time and staffing allows on a set bi-weekly slate. Theatre time cuts have decreased the operative capacity from 4 to 2 cases per week which greatly limits SB referrals. Limited postoperative care beds also contribute to a backlog.
Surgically correctable anomalies appear to be much more prevalent in males. However, many sources have shown a higher occurrence of SB amongst females.15 Normal vaginal deliveries were more common in our setting. Some sources support elective caesarean section for certain anomalies, however, surgical delivery is not currently considered standard of care in either AWD or SB. Delivery route does not seem to affect overall outcome.16 Although not shown in our study, an association exists between congenital malformations and premature delivery.17 Due to the scarcity of early ultrasound to assist accurate gestational age determination, estimated maturity may have been incorrect. Gastroschisis is strongly associated with younger maternal age.18 Certain maternal age groups also appear to have added risk for NTDs especially 40 years and older, and young mothers less than 19 years old.19
Antenatal clinic attendance (98%) was much higher than the average antenatal coverage for the region (76%).20 Although this high attendance is encouraging, it does indicate missed opportunities for antenatal diagnosis and referral. Mnguni et al. observed an antenatal diagnosis in only 1.90% despite reasonable antenatal attendance.9 Although routine screening antenatal ultrasound in low risk populations is not standard practice, it is encouraged as part of World Health Organization recommendations.21 Nevertheless, antenatal diagnosis is only considered prudent if this could improve outcomes. Delivery at a tertiary facility with rapid access to neonatal critical care, anaesthetic and appropriate surgical services would be necessary, but access to emergency interfacility neonatal transfer services are limited in KwaZulu-Natal.22 Foetal surgery for NTDs, whether through open or endoscopic technique, is not currently available in our setting.23 There are no current surgical options for foetal surgery in cases of AWDs.24
The advent of COVID-19 did not have a significant effect on waiting times. The pandemic required redistribution and reallocation of resources, resulting in delays in surgical service delivery, including emergency neonatal surgery.25 Although disruptions to paediatric surgical services were experienced in both high-income countries (HIC) and low- to middle-income countries (LMIC), HIC were able to recover services quickly, whereas in sub-Saharan Africa emergency surgery dropped by 23% and congenital surgery decreased by 34.7%.26
Hydrocephalus is the most common long term comorbidity in the majority of patients with SB.27 The lower percentage in our population is likely due to underreporting in clinical notes, possible inaccuracies in bedside ultrasound interpretation combined with limited access to computerised tomography. Associated musculoskeletal comorbidities are expected. However, no urogenital conditions, commonly associated with SB, were seen. These were likely underreported. We reported "dysmorphism" as a problem included in the clinical records and acknowledge that SB or AWDs are dysmorphisms and that the additional nonspecific dysmorphic features should be interpreted with caution. Interestingly, Waardenburg syndrome appears to increase the risk of MMC, albeit a rare occurrence.28 Congenital heart defects are fairly common both in SB and AWDs, although mostly associated with omphaloceles.29 Omphalocele and Beckwith-Wiedemann syndrome are also closely linked.30
It is likely that more patients with AWD and SB were delivered at district facilities but did not survive to reach our facility, and this could explain the relatively higher chance of mortality for inborn babies. Clinical instability prevented the timely transfer of two patients, and limited tertiary capacity also contributed. Mortality may have been higher if tertiary outcomes were included.
Complications were common and even more prevalent in cases of open SB. This may be related to the longer delays. The presence of a leaking MMC likely prompted referral from a base hospital to our facility, explaining the high prevalence of this complication amongst SB cases. In addition to delays in MMC repair, the technique of delivery as well as nursing and handling of the defects contribute to leaking. Some SB patients may have remained at the primary hospital awaiting direct referral to the tertiary facility and were thus not included in our study. Cerebrospinal fluid leaks likely contributed to late neonatal infections.9 Similarly, prolonged dependence on total parenteral nutrition, coupled with an open defect probably contributed to infections in AWD cases. Only one case of confirmed meningitis was reported, and this is likely underreported. Seizures may have been more common as clinical signs lack sensitivity for subtle neonatal seizures. Paraplegia is commonly associated with open SB and was also likely underrepresented.9 Respiratory failure was more common in cases of open AWD, probably due to a combination of impaired diaphragmatic movement and lung development.31
Limitation
Our study was limited by a small sample size. Difficulties in patient record retrieval greatly hampered our data collection, particularly during 2018 and 2019. In addition, we only included patients presenting to our facility and our findings are thus likely under-representative of disease burden. No follow-up data were available.
Strengths
We used easily diagnosed and potential bellwether conditions for two distinct surgical defects. This study can be reproduced at other facilities to improve our body of knowledge on access to care.
Conclusion and recommendations
Newborns with open SB or AWDs experience marked delays in reaching definitive care. This is more pronounced for cases of SB and was not influenced by the pandemic. Tertiary hospital capacity is the most important limiting factor. Many complications arise and may contribute to long-term morbidity. Our study demonstrated a relatively high mortality, which may have been even higher if primary and tertiary facility data were also included.
Investment in more rapid transfers via air ambulance may be beneficial, however, tertiary level capacity will likely remain the most important limiting factor. Some neonatal surgical services, as part of an outreach programme, may be provided at the regional hospital level. This may be more prudent while tertiary services remain scarce. Further investigation of long-term morbidities through later follow-up should also be considered.
Acknowledgements
We would like to thank the administrative team for assisting in clinical record retrieval. Thanks are also extended to Ms D Pearce for her assistance with the electronic database.
Conflict of interest
The authors declare no conflict of interest.
Funding source
None.
Ethical approval
The provincial department of health and local institutional approval was obtained. Ethical approval was granted by the University of KwaZulu-Natal's Biomedical Research Ethics Committee (BREC/00003984/2022) as well as the hospital's ethics committee.
ORCID
R Vosloo https://orcid.org/0000-0002-6861-5381
G Wyer https://orcid.org/0009-0002-1619-3401
L Naidoo https://orcid.org/0009-0001-2840-5902
B Enicker https://orcid.org/0000-0003-1285-3046
AG Maharaj https://orcid.org/0000-0003-2501-564X
NC Kapongo https://orcid.org/0000-0003-3311-7216
REFERENCES
1. Sitkin NA, Ozgediz D, Donkor P, Farmer DL. Congenital anomalies in low- and middle-income countries: the unborn child of global surgery. World J Surg. 2015;39(1):36-40. https://doi.org/10.1007/s00268-014-2714-9. [ Links ]
2. World Health Organization. December 2020. Birth defects fact sheet. Available from: https://www.who.int/news-room/fact-sheets/detail/birth-defects. Accessed 29 December 2022. https://doi.org/10.1007/978-1-4612-6150-6_8. [ Links ]
3. Saib MZ, Dhada BL, Aldous C, Malherbe HL. Observed birth prevalence of congenital anomalies among live births at a regional facility in KwaZulu-Natal Province, South Africa. PLoS One. 2021;16(8)):1-16. https://doi.org/10.1371/journal.pone.0255456. [ Links ]
4. Kirby RS. The prevalence of selected major birth defects in the United States. Semin Perinatol. 2017;41(6):338-44. https://doi.org/10.1053/j.semperi.2017.07.004. [ Links ]
5. Prefumo F, Izzi C. Fetal abdominal wall defects. Best Pract Res Clin Obstet Gynaecol. 2014;28(3):391-402. https://doi.org/10.1016/j.bpobgyn.2013.10.003. [ Links ]
6. Wright NJ, Sekabira J, Ade-Ajayi N. Care of infants with gastroschisis in low-resource settings. Semin Pediatr Surg. 2018;27(5):321-6. https://doi.org/10.1053/j.sempedsurg.2018.08.004. [ Links ]
7. Theron N, Joubert G, Henderson BD. Neural tube defects in the free state province from 2012 to 2016. Is there an increase? S Afr J HIV Med. 2020;21(1):1-7. https://doi.org/10.4102/sajhivmed.v21i1.1134. [ Links ]
8. Dewan MC, Baticulon RE, Ravindran K, et al. Pediatric neurosurgical bellwether procedures for infrastructure capacity building in hospitals and healthcare systems worldwide. Child's Nerv Syst. 2018;34(10):1837-46. https://doi.org/10.1007/s00381-018-3902-y. [ Links ]
9. Mnguni MN, Enicker BC, Madiba TE. A perspective in the management of myelomeningocele in the KwaZulu-Natal Province of SouthAfrica. Child's Nerv Syst. 2020;36(7):1521-7. https://doi.org/10.1007/s00381-020-04506-9. [ Links ]
10. National Government of South Africa. Municipalities of South Africa - KwaZulu-Natal Municipalities. 2023. Available from: https://municipalities.co.za/municipalities/type/2/district. Accessed 03 January 2023. [ Links ]
11. Sekabira J, Hadley GP. Gastroschisis: a third world perspective. Pediatr Surg Int. 2009;25(4):327-9. https://doi.org/10.1007/s00383-009-2348-4. [ Links ]
12. Global PaedSurg Research Collaboration. Mortality from gastrointestinal congenital anomalies at high-income countries: a multicentre, international, prospective cohort study. 2021;398:325-39. https://doi.org/10.1016/S0140-6736(21)00767-4. [ Links ]
13. Ikol KM, Saula PW, Gisore P, Mvungu E, Mwangi HR. Outcomes of neonates requiring surgical interventions in Eldoret. Ann African Surg. 2019;16(1):20-5. https://doi.org/10.4314/aas.v16i1.5. [ Links ]
14. Skarsgard ED. Management of gastroschisis. Curr Opin Pediatr. 2016;28(3):363-9. https://doi.org/10.1097/MOP.0000000000000336. [ Links ]
15. Muzumdar D, Hawaldar A, Bhambhere S, et al. Open neural tube defects in covid-19: an analysis of 26 neonatal patients in a tertiary care center. J Pediatr Neurosci. 2021;16(1):5-10. https://doi.org/10.4103/jpn.JPN_126_21. [ Links ]
16. Wataganara T, Grunebaum A, Chervenak F, Wielgos M. Delivery modes in case of fetal malformations. J Perinat Med. 2017;45(3):273-9. https://doi.org/10.1515/jpm-2015-0364. [ Links ]
17. Swanson JR, Sinkin RA. Early births and congenital birth defects: A complex interaction. Clin Perinatol. 2013;40(4):629-44. https://doi.org/10.1016/j.clp.2013.07.009. [ Links ]
18. Rittler M, Campana H, Ermini ML, et al. Gastroschisis and young mothers: What makes them different from other mothers ofthe same age? Birth Defects Res. 2015;103(6):536-43. https://doi.org/10.1002/bdra.23374. [ Links ]
19. Vieira A, Castillo TS. Maternal age and neural tube defects: evidence for a greater effect in spina bifida than in anencephaly. Rev Med Chil. 2005;Jan 133(1):62-70. https://doi.org/10.4067/S0034-98872005000100008. [ Links ]
20. Massyn N, Day C, Ndlovu N, Padayachee T, editors. District health barometer 2019/20. Durban: Health Systems Trust; December 2020. [ Links ]
21. World Health Organization. Recommendations on antenatal care for a positive pregnancy experience: ultrasound examination. Highlights and key messages from the World Health Organization's 2016 Global Recommendations; 2018. Available from: http://apps.who.int/ir. http://apps.who.int/iris/bitstream/10665/259946/1/WHO-RHR-18.01-eng.pdf. [ Links ]
22. Ashokcoomar P, Naidoo R. An analysis of inter-healthcare facility transfer of neonates within the eThekwini Health District of Kwazulu-Natal, South Africa. S Afr Med J. 2016;106(5):514-8. https://doi.org/10.7196/SAMJ.2016.v106i5.8554. [ Links ]
23. Dewan MC, Wellons JC. Fetal surgery for spina bifida. J Neurosurg Pediatr. 2019;24(2):105-14. https://doi.org/10.3171/2019.4.PEDS18383. [ Links ]
24. Durmaz LO, Brunner SE, Meinzer A, Krebs TF, Bergholz R. Fetal surgery for gastroschisis - A review with emphasis on minimally invasive procedures. Children. 2022;9(3):1-22. https://doi.org/10.3390/children9030416. [ Links ]
25. Gunadi, Idham Y, Paramita VMW, et al. The impact of COVID-19 pandemic on pediatric surgery practice: A cross-sectional study. Ann Med Surg. 2020;59:96-100. https://doi.org/10.1016/j.amsu.2020.09.020. [ Links ]
26. Mazingi D, Shinondo P, Ihediwa G, et al. The impact of the COVID-19 pandemic on paediatric surgical volumes in Africa: A retrospective observational study. J Pediatr Surg. 2022. https://doi.org/10.1016/j.jpedsurg.2022.10.047. [ Links ]
27. Blount J, Maleknia P, Hopson B, Rocque B, Oakes W. Hydrocephalus in spina bifida. Neurol India. 2021;69(8):S363-S367. https://doi.org/10.4103/0028-3886.332247. [ Links ]
28. Hart J, Miriyala K. Neural tube defects in Waardenburg syndrome: A case report and review of the literature. Am J Med Genet Part A. 2017;173(9):2472-7. https://doi.org/10.1002/ajmg.a.38325. [ Links ]
29. Gibbin C, Touch S, Broth RE, Berghella V. Abdominal wall defects and congenital heart disease. Ultrasound Obstet Gynecol. 2003;21(4):334-7. https://doi.org/10.1002/uog.93. [ Links ]
30. Spivey PS, Bradshaw WT. Recognition and management of the infant with Beckwith-Wiedemann Syndrome. Adv Neonatal Care. 2009;9(6):279-84;quiz 285. https://doi.org/10.1097/ANC.0b013e3181c2003f. [ Links ]
31. Obayashi J, Tanaka K, Koike J, et al. Does a large abdominal wall defect affect lung growth? J Pediatr Surg. 2016;51(12):1972-5. https://doi.org/10.1016/jopedsurg.2016.09.022. [ Links ]
 Correspondence:
Correspondence:
R Vosloo
Email: drruanvosloo@icloud.com














