Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Surgery
versión On-line ISSN 2078-5151
versión impresa ISSN 0038-2361
S. Afr. j. surg. vol.62 no.1 Cape Town 2024
http://dx.doi.org/10.36303/SAJS.00212
PAEDIATRIC SURGERY
Endoscopic findings in children born with oesophageal atresia in an academic unit in South Africa
C de VosI, II; N FourieI; B BanieghbalI; PT SchubartIII; D SidlerIV; P GoussardII
IDivision of Paediatric Surgery, Department of Surgical Sciences, Stellenbosch University, South Africa
IIDepartment of Paediatric and Child Health, Stellenbosch University, South Africa
IIIDivision of Anatomical Pathology, Department of Pathology, Stellenbosch University, South Africa
IVCentre for Medical Ethics and Law, Stellenbosch University, South Africa. Caringfor Children: Paediatric Surgery in the Department of Paediatrics and Health, Red Cross War Memorial Children's Hospital, University of Cape Town, South Africa
ABSTRACT
BACKGROUND: Oesophageal atresia (OA) is one of the most common congenital gastrointestinal (GI) abnormalities. Due to advances in multidisciplinary care, early prognosis has improved with emphasis shifting to the long-term impact of this disease. Literature suggests a higher incidence of Barrett's and eosinophilic oesophagitis in these children, with an increased risk of oesophageal carcinoma. Guidelines for adults born with OA include routine endoscopy and lifelong screening of the upper gastrointestinal tract (GIT). Despite this, uncertainty remains regarding the necessity and frequency of endoscopic surveillance for children born with OA. We describe our endoscopic findings in children born with OA
METHOD: A prospective analytic cohort study was undertaken, which included all children born with OA, that were followed-up in our unit between 2020 and 2022. History regarding feeding and GI symptoms were documented after which an endoscopy was performed
RESULTS: During the study period, 37 endoscopies were performed in patients born with OA at a median age of 25 months. The most common clinical appearance on endoscopy was anastomotic strictures followed by oesophagitis. Twelve patients had biopsies taken, with abnormal histology in all but one patient. The most common histological finding was oesophagitis with lymphocytes and chronic gastritis. Two patients had Helicobacter Pylori infection, and one had findings suggestive of eosinophilic oesophagitis
CONCLUSION: All patients with a clinical indication for an endoscopy had abnormal clinical or histological findings, thus concurring with the literature in highlighting the need for regular endoscopy. We recommend regular clinical follow-up and endoscopic surveillance if clinically indicated for children born with OA
Keywords: oesophageal atresia, endoscopy, morbidity, long-term outcomes
Introduction
Oesophageal atresia (OA) is one of the most common congenital gastrointestinal (GI) abnormalities requiring surgery early in life. This complex disease is rare and remains a major therapeutic challenge in most centres.1 Fortunately, due to advances in collaborative multidisciplinary care, the early prognosis of these children has improved, with survival reaching more than 90%.2 The emphasis is now on the impact of the disease both postoperatively and on the long-term outcome of these patients.
The GI system is one of the organ systems most affected in patients born with OA. Common symptoms include dysphagia, gastro-oesophageal reflux disease (GORD), oesophagitis, oesophageal strictures, and Barrett's oesophagus (BE).3-5 Current literature suggests that the incidence of BE and eosinophilic oesophagitis (EoE) is higher in children born with OA.6 Furthermore, two long-term follow-up studies have shown an increased risk of both BE and oesophageal carcinoma at a relatively young age in patients born with OA.25
Guidelines for adults born with OA recommend routine endoscopy and lifelong screening of the upper GIT.5 Despite this and the potential long-term GI complications, uncertainty remains regarding the necessity and frequency of endoscopic surveillance for children born with this disease.
We describe endoscopic findings of children born with OA in our unit.
Method
A prospective analytic cohort study was undertaken. All children born with OA, who presented to our unit between 2020 and 2022 with a clinical indication for an endoscopy were included in the study.
Patients were admitted, and an endoscopy with/without oesophageal dilatation or biopsies were performed by one of the three paediatric surgery consultants in our unit. Indications for endoscopy included: surveillance endoscopy (for patients with no previous endoscopy done), symptoms suggestive of dysphagia, foreign body ingestion, GORD, and follow-up endoscopy after a previous abnormal endoscopy with/without biopsy. All endoscopies were done in theatre under general anaesthesia.
The presenting symptom and reason for endoscopy, the patients' age at the time of endoscopy, as well as the admission weight and height of the patients, was documented by the primary researcher on a standardised form.
All specimen biopsies were stored in formalin and taken to the laboratory where they underwent routine H&E staining with further special investigations (as per norm) if indicated.
Ethical and statistical considerations
All information was anonymised. Clinical history, endoscopic findings and histological results were recorded on a "REDCap" database.7 Descriptive analysis with medians and interquartile ranges (IQR), numbers, and percentages was performed. P-values were not calculated.
Ethical principles were adhered too. Both ethical (HREC reference nr: S20/10/260) and institutional approval (WC_202103_006) were obtained before the onset of the study.
Results
During the study period (2020-2022) 22 patients born with OA were followed-up in our unit. Of these, 16 (73%) had a clinical indication for endoscopy and received a total of 37 endoscopic procedures. The majority (n = 10, 63%) were male with a median age at the time of endoscopy of 25 months (IQR 3.5-75.5). Our patients' median weight and height at the time of endoscopy were 9 kg (IQR 5-13.5) and 92 cm (IQR 65-111.5), respectively. The rest of the demographic information collected is summarised in Table I.
Presenting gastrointestinal symptoms
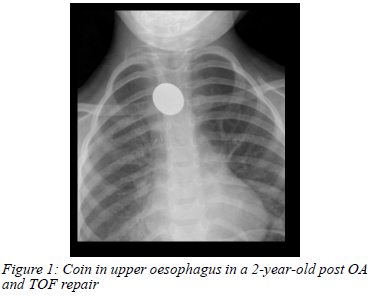
Thirteen (35%) endoscopic cases had complaints of dysphagia, eight (62%), of which were for solid food only. Thirty (81%) endoscopic cases were on a proton pump inhibitor (PPI) at the time of endoscopy. Two (5%) patients (a 2-year- and a 3-year-old) had a history of foreign body ingestion that was confirmed on chest radiograph and found to be at the anastomotic site (Figure 1).
Endoscopic findings
Indications for the 37 endoscopies included - surveillance endoscopy in six (16%) cases for children born with OA with no previous endoscopy, four (11%) cases with symptoms suggestive of gastro-oesophageal reflux and 21 (57%) cases with symptoms suggestive of an oesophageal stricture or a follow-up endoscopy after previous dilatation for a stricture. Of the remaining six cases, two (5%) had a history of foreign body ingestion, one (3%) had symptoms of reflux with a history of haematemesis post colonic interposition and three (8%) were follow-up endoscopies after previous abnormal endoscopic and/or histology findings which included - a previous bleeding ulcer (n = 1, 3%), follow-up endoscopy after a previous diagnosis of EoE (n = 1, 3%) and follow-up endoscopy for a patient with severe oesophagitis and a diverticulum (n = 1, 3%). All the endoscopic investigations except for one patient (who had two scopes performed by the adult gastroenterologists) were done by one of the three paediatric surgeons in our unit. Abnormalities were found in all the endoscopic cases. The most common clinical appearance as documented by the surgeons was an anastomotic stricture (n = 27, 57%) followed by oesophagitis (n = 12, 32%), macroscopic evidence suggestive of a fungal infection (n = 7, 19%) and clinical GI reflux (n = 6, 16%). Gastritis was reported in five (14%) cases, and in another five (14%) cases a diverticulum was visible. One (3%) case had evidence of a resolving gastric ulcer on the first endoscopy and a healing ulcer at follow-up one month later.
Twenty-one (57%) of the 37 cases presented with an oesophageal stricture, 20 of which were dilated at the time of endoscopy (19 with rigid bougie dilators and one with a balloon dilator). All the strictures were documented to be at the site of previous anastomosis.
Histology results
Ten (63%) of the sixteen patients had a clinical indication for histological investigations at the time of endoscopy resulting in a total of 12 (32% of all cases) biopsies with one patient needing two follow-up biopsies. The most common histological findings were oesophagitis with lymphocytes (n = 7, 58%) and chronic gastritis in four (33%) cases (Figure 2).
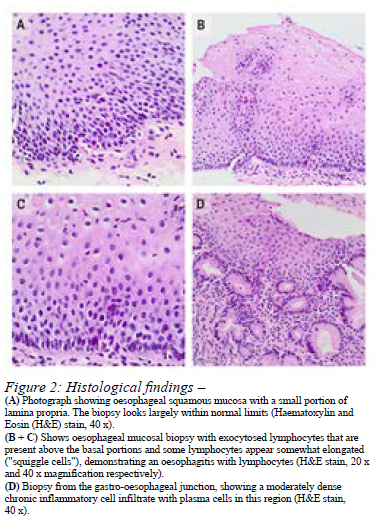
In one eight-month-old patient, there was oesophagitis with lymphocytes in the distal oesophagus and eosinophilia in the mid oesophagus. After oral PPI treatment, a further scope was done 14 months later, which confirmed oesophagitis with lymphocytes in the proximal, middle, and distal oesophagus, but no EoE. A repeat endoscopy with biopsies was done for the same patient six months later and this time the distal oesophagus had chronic oesophagitis with Helicobacter pylori (H. pylori) activity for which she was treated accordingly. One other patient was also diagnosed with H. pylori activity. The histology for the remaining nine patients is discussed in Table II.
All the patients were followed-up at outpatients and started on appropriate treatment if needed. Follow-up scopes were scheduled for patients if indicated. No surgical or anaesthetic-related complications were reported during or after any of the endoscopy procedures.
Discussion
The role of endoscopic surveillance post-OA repair in children is still controversial. There are increasing reports in the literature of children with OA who present with BE, EoE, and oesophageal carcinoma.2,5,6 In our study all children born with OA with either a clinical indication for endoscopy or those who did not have any previous endoscopies (and had a surveillance endoscopy) had abnormal endoscopic findings, which highlights the need for further research and treatment protocols for these children.
One of the most common causes of morbidity in patients born with OA is anastomotic strictures, with an incidence between 32-59%.9 Friedmacher et al. recently published their longitudinal study on postoperative complications and their impact on the long-term GI function of patients born with OA.1 In their study about 70% of cases presented with anastomotic strictures requiring three dilatations; this is higher than the 57% of cases in our cohort. Despite this, recommendations are that routine screening and dilatation should not be done for children post-OA repair and that anastomotic strictures should be treated only if the patients are symptomatic.9,10
GORD and lymphocytic oesophagitis have a higher incidence in children born with OA.11 Seven (19%) of the cases biopsied in our study showed oesophagitis with lymphocytes on histological evaluation. Despite mild to moderate symptoms, most of them were on a PPI prior to endoscopy. Our results concluded that all but one patients had abnormal histological results raising the question for the need to establish a regular endoscopy follow-up programme for OA patients. Fourteen (31%) patients who underwent endoscopy in a Montreal Study published by Castilloux et al. had oesophagitis, slightly more than in our cohort.12 They had an additional 16 (36%) cases that presented with gastric metaplasia, where we had none in our study.12
EoE is a histological diagnosis characterised by the presence of eosinophils in the mucosa and submucosa of the oesophagus.13 EoE has had an increased recognition and global incidence. Patients can present with GORD and dysphagia.13 Additionally, EoE has an increased prevalence in children born with OA and raises the question of whether patients with OA should be screened for this condition, especially if their symptoms persist despite maximum medical treatment.14 We only had one patient with eosinophils present on biopsy but Kassabian et al. presented four patients (ages ranging from 9-16 years) post-OA repair with EoE, and recommended that patients post-OA repair with refractory GORD and dysphagia be screened for this condition.14 Gorter et al. published two cases of patients (6 and 12 years old) post-OA who presented with EoE.13 Horning et al. stated that in patients with OA early recognition and treatment of EoE can prevent the incidence of strictures and the need for dilatation.6 The European Society for Paediatric Gastroenterology, Hepatology and Nutrition/ North American Society For Paediatric Gastroenterology, Hepatology & Nutrition (ESPGHAN/ NASPGHAN) guidelines recommend exclusion of EoE in all patients born with OA and presenting with refractory dysphagia, GORD, coughing or choking, or recurrent strictures, despite maximum medical treatment, before antireflux surgery.10
Although we did not have any patients with features suggesting BE, the current literature does indicate that the prevalence is higher in children born with OA. The American College of Gastroenterology defines BE as a change of any length of the epithelium of the oesophagus.15 It can be recognised at endoscopy, and the diagnosis is confirmed by biopsies and histological evaluation. Tan et al. published the first report of a patient who had both EoE and BE, highlighting the need for early endoscopic surveillance.11 Conner et al. found 306 patients born with OA (children and adults) with BE in their review article, with a pooled prevalence of 6.4%.4 They also reported that 1.4% of patients in their review (children and adults) developed squamous cell oesophageal carcinoma. Hsieh et al. retrospectively reviewed patients born with OA and followed-up at three academic centres.16 Twelve (1.7%) (children and adolescents) were diagnosed with intestinal metaplasia at a median age of 10.9 years, the youngest being 2 years old, suggesting early endoscopic surveillance for OA (especially GORD-prone) patients.
The last part of the ESPGHAN-NASPGHAN guidelines for patients with OA recommends continued endoscopic screening with the transition from paediatric to adult care.10 They found eight case reports of oesophageal cancer (adenocarcinoma and squamous cell carcinoma) during their literature review. Vergouwe et al. presented four patients with OA who developed cancer as adults (the youngest patient being 36 years old).5 They reported several longterm studies which showed an increased risk of both BE and oesophageal carcinoma at an early age in patients with OA and concur with the ESPGHAN-NASPGHAN guidelines that routine endoscopy should be performed in adults. The ESPGHAN-NASPGHAN guidelines recommended endoscopic surveillance for adults with OA every 5-10 years or when they have any new symptoms or when their chronic symptoms get worse.10
Our results suggest that an endoscopic investigation is indicated in the presence of any symptoms. Abnormal clinical appearances were documented in all thirty-seven endoscopic cases. Limitations of the study include the small cohort and single centre results. We recommend a larger multi-centre study in the future.
Conclusion
Children born with OA often have long-term GI morbidities, ranging from mild dysphagia to oesophageal strictures. This study confirmed that all our patients with a clinical indication for an endoscopy had abnormal clinical and/ or histological findings, thus concurring with the literature review in highlighting the need for regular endoscopy.
After the initial OA repair, we recommend a monthly clinical follow-up with specific questions and clinical examination (including weight and height for age) for the first few months post-discharge from the hospital. Afterward, this can be increased to six-monthly and later to annual visits depending on the clinical response of the patient.
Due to potential complications associated with general anaesthesia (and constraints on theatre availability), we would recommend endoscopic surveillance in children with dysphagia, refractory GORD despite maximum medical treatment, failure to thrive despite adequate nutritional support, recurrent respiratory symptoms or as surveillance at least every 3-5 years. With transitioning from paediatric care to adult care we would support follow-up as recommended by the ESPGHAN-NASPGHAN guidelines with endoscopy and biopsies every 5-10 years or when either new symptoms present, or regular symptoms worsen.
Conflict of interest
None declared.
Funding source
This research did not receive any specific grant from funding agencies in the public, commercial or non-profit sectors.
Ethical approval
The protocol for this article conforms to the provisions of the Declaration of Helsinki (1995) and has been approved by the Stellenbosch University Health Research Ethics Committee (S20/10/260).
ORCID
C de Vos https://orcid.org/0000-0002-5024-5693
N Fourie https://orcid.org/0000-0004-0635-423X
B Banieghbal https://orcid.org/0000-0003-4203-7904
PT Schubart https://orcid.org/0000-0003-4422-7349
P Goussard https://orcid.org/0000-0003-1146-1307
REFERENCES
1. Friedmacher F, Kroneis B, Huber-Zeyringer A, et al. Postoperative complications and functional outcome after esophageal atresia repair: Results from longitudinal single-centre follow-up. J Gastrointest Surg. 2017;21(6):927-35. https://doi.org/10.1007/s11605-017-3423-0. [ Links ]
2. Koivusalo AI, Pakarinen MP, Lindahl HG, Rintala RJ. Endoscopic surveillance after repair of oesophageal atresia - longitudinal study in 209 patients. J Pediatr Gastroenterol Nutr. 2016;62(4):562-6. https://doi.org/10.1097/MPG.0000000000000972. [ Links ]
3. Roberts K, Karpelowsky J, Fitzgerald DA, Soundappan SS. Outcomes of oesophageal atresia and trachea-oesophageal fistula repair. J Paediatr Child Health. 2016;52(7):694-8. https://doi.org/10.1111/jpc.13211. [ Links ]
4. Connor MJ, Springford LR, Kapetanakis VV, Giuliani S. Oesophageal atresia and transitional care - Step 1: a systematic review and meta-analysis of the literature to define the prevalence of chronic long-term problems. Am J Surg. 2015;209(4):747-59. https://doi.org/10.1016/j.amjsurg.2014.09.019. [ Links ]
5. Vergouwe FW, Gottrand M, Wijnhoven BP, et al. Four cancer cases after oesophageal atresia repair: Time to start screening the upper gastrointestinal tract. World J Gastroenterol. 2018;24(9):1056-62. https://doi.org/10.3748/wjg.v24.i9.1056. [ Links ]
6. Horning A, Homan M, Krishnan U. Eosinophilic oesophagitis in oesophageal atresia. Front Paediatr. 2019;7:497. https://doi.org/10.3389/fped.2019.00497. [ Links ]
7. Vanderbilt University. REDCap technical overview introduction REDCap infrastructure - best practices and dependencies; 2017. p. 1-6. [ Links ]
8. Gross RE. Atresia of the oesophagus. In: Saunders W, editor. Surgery of infancy and childhood. Philadelphia: WB Saunders; 1953. p. 76. [ Links ]
9. Van Der Zee DC, Rolle U, Van Herwaarden-Lindeboom M, et al. Anastomotic Strictures after oesophageal atresia repair - incidence, investigations, and management, including treatment of refractory and recurrent strictures; 2017. p. 5. [ Links ]
10. Krishnan U, Mousa H, Dall'Oglio L, et al. ESPGHAN-NASPGHAN guidelines for the evaluation and treatment of gastrointestinal and nutritional complications in children with oesophageal atresia-tracheoesophageal fistula. J Pediatr Gastroenterol Nutr. 2016;63(5):550-70. https://doi.org/10.1097/MPG.0000000000001401. [ Links ]
11. Tan LZ, Gifford AJ, Clarkson CM, Henry GM, Krishnan U. Barrett's oesophagus and eosinophilic esophagitis in a young paediatric patient with oesophageal atresia. J Pediatr Surg Case Rep. 2015;3(7):272-5. https://doi.org/10.1016/j.epsc.2015.05.004. [ Links ]
12. Castilloux J, Bouron-Dal Soglio D, Faure C. Endoscopic assessment of children with oesophageal atresia - lack of relationship of oesophagitis and oesophageal metaplasia to symptomatology. Can J Gastroenterol. 2010;24(5):312-6. https://doi.org/10.1155/2010/902847. [ Links ]
13. Gorter RR, Heij HA, Voorn JP Van Der, Kneepkens CMF. Eosinophilic oesophagitis after oesophageal atresia - is there an association? Case presentation and literature review. J Pediatr Surg. 2012;47(6):e9. https://doi.org/10.1016/j.jpedsurg.2012.01.079. [ Links ]
14. Kassabian S, Baez-Socorro V, Sferra T, Garcia R. Eosinophilic oesophagitis in patients with oesophageal atresia and chronic dysphagia. World J Gastroenterol. 2014;20(47):18038-43. https://doi.org/10.3748/wjg.v20.i47.18038. [ Links ]
15. Shalauta MD, Saad R. Barrett's oesophagus. Exp Clin Gastroenterol. 2004;(6):134-8. [ Links ]
16. Hsieh H, Frenette A, Michaud L, et al. Intestinal metaplasia of the oesophagus in children with oesophageal atresia. J Pediatr Gastroenterol Nutr. 2017;65(1):e1-e4. https://doi.org/10.1097/MPG.0000000000001558. [ Links ]
 Correspondence:
Correspondence:
C de Vos
Email: cdevos@sun.ac.za or devos.corne@gmail.com














