Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Surgery
versión On-line ISSN 2078-5151
versión impresa ISSN 0038-2361
S. Afr. j. surg. vol.61 no.4 Cape Town 2023
http://dx.doi.org/10.36303/sajs.4120
CASE REPORT
Small bowel metastasis from embryonal rhabdomyosarcoma of the extremity - a case report
P JoubertI; M MihalikI, II
IDepartment of Surgery, New Somerset Hospital, South Africa
IIDepartment of General Surgery, Groote Schuur Hospital, South Africa
ABSTRACT
Rhabdomyosarcoma is the most common soft tissue tumour in children and adolescents, but extremely rare in adults with comparatively worse outcomes. Metastatic disease is not uncommon, but intra-abdominal metastases are exceedingly rare. We report an unusual case of ileal metastases from an upper extremity rhabdomyosarcoma in a 17-year-old male who presented with abdominal pain during a routine follow-up visit. Laparotomy and ileocecectomy for a perforated ileal mass confirmed metastatic embryonal rhabdomyosarcoma with 1 out of 14 positive lymph node metastases. This case demonstrates that, although rare, intra-abdominal metastases should be considered when patients with a rhabdomyosarcoma present with abdominal complaints.
Keywords: rhabdomyosarcoma, metastasis, adolescent, embryonal
Case report
A previously well 17-year-old male was referred for investigation of a left forearm mass which had been progressively enlarging over 1 year. There was associated significant unintentional weight loss as well as intermittent pain and paraesthesia in the affected arm. He was wasted with axillary and brachial lymphadenopathy, as well as radial and ulnar nerve palsies. Forearm magnetic resonance imaging (MRI) supported the differential of a rhabdomyosarcoma with neural encasement and a core needle biopsy (CNB) confirmed features in keeping with rhabdomyosarcoma.
During a routine outpatient follow-up for his results, he complained of severe lower abdominal pain, loss of appetite and fever. A poorly defined, tender mass with localised peritonitis was palpable in the right lower quadrant.
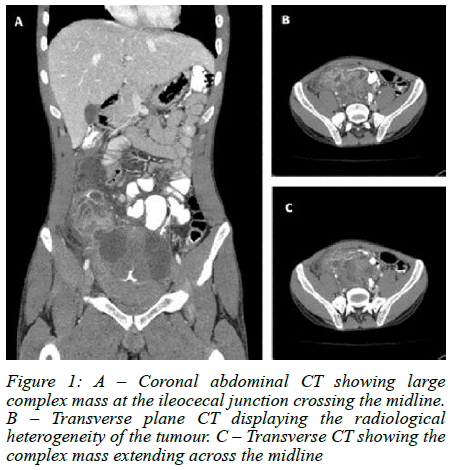
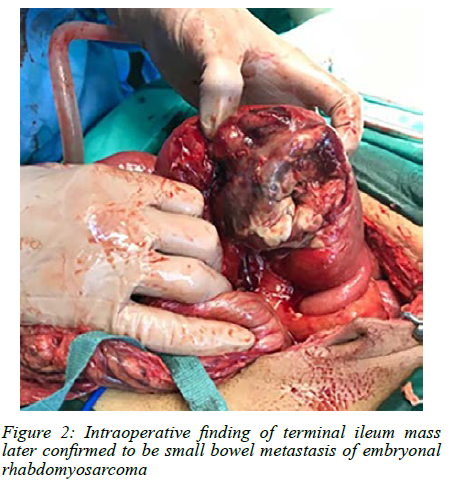
Urgent computer tomography (CT) imaging suggested a complex pelvic mass, likely ileocaecal in origin (Figure 1). He underwent emergency laparoscopy converted to laparotomy which revealed a complex perforated terminal ileum mass 10 cm from the ileocaecal junction (Figure 2). An ileocecectomy with primary anastomosis was performed. Histology confirmed metastatic embryonal rhabdomyosarcoma with 1 out of 14 mesenteric lymph nodes involved. Our patient had an uneventful postoperative course and was discharged in a stable condition. He is currently undergoing palliative chemo-radiotherapy for spinal, liver and lung metastases.
Discussion
Rhabdomyosarcoma (RMS) is a high grade primitive mesenchymal cell tumour thought to arise due to aberrations in myogenic proliferation and differentiation.1 It is the most common soft tissue tumour (STT) in the childhood and adolescent populations, accounting for 50% of all soft tissue tumours.2 Whilst it is third only to Wilms' tumour and neuroblastoma, it is still rare, accounting for only 4.5% of childhood cancers overall.3 Histological subtypes of RMS are widely classified, but broadly there are 2 major variants: embryonal rhabdomyosarcoma (ERMS) and alveolar rhabdomyosarcoma (ARMS), with pleomorphic RMS, ganglionic differentiation and RMS not otherwise specified (NOS) seen as separate entities.2
Seen primarily as an early childhood cancer with almost one third being diagnosed before the age of 6, RMS has a bimodal distribution for presenting age with a second peak in adolescence.4,5 The incidence decreases dramatically with age and is associated with significant clinical and histological heterogeneity.6 ERMS is by far the more common subtype (70%) in childhood, and it can be further classified as conventional, spindle cell and botryoid subtypes.7 ERMS and ARMS are more common in children with very few cases noted in adults, and typically only present between the ages of 22 and 40 years. In the adolescent population ARMS has been deemed to be more likely.4 Pleomorphic RMS and RMS NOS is specifically more common in adults and has been described as being more closely related to high grade soft tissue sarcoma.6 Our patient presented to us in adolescence with metastatic ERMS which leads to a consideration of potential overlap between the subtypes in age population and histology. The aetiology and development of different histological variants in different age groups is still largely unknown. Besides genetic syndromes which are generally associated with childhood presentation, the complexity of mechanisms associated with developing RMS has questioned the differences in histology.8
RMS has a wide variety of primary sites of disease with variation dependant on the different histological subtypes and age of presentation.4 Broadly, the most common sites of primary tumours are head and neck (35%), genitourinary organs and the extremity. Intra-abdominal and retroperitoneal primary sites are rarer and there are notable differences in primary sites of disease between adult and child populations.8 Tumours located in the trunk, the upper and lower limbs occur more frequently in adolescents and often demonstrate ARMS histology.4 RMS metastases occur via the haematogenous route primarily to the lung, bones, liver, brain and regional lymph nodes;9 the latter are often present with primary tumours of the extremity.9 Intra-abdominal metastases are extremely rare in paediatric, adolescent and adult populations with few cases described.10 Several case reviews have described adults with advanced pleomorphic RMS subtypes who presented with intussusception as the only feature of metastasis.10 This deems our case unusual as he was diagnosed with primary ERMS of the extremity, which is a well described primary site in the paediatric population and early adulthood, with confirmed metastatic ERMS of the small bowel, complicated by perforation.
Prognostically, outcomes associated with RMS are generally favourable, with a 5-year survival of 70-80% in children. However, the 5-year prognosis is comparatively less favourable in adults and adolescents.11 The difference in prognosis is associated with histological subtype, primary site and size, lymphovascular involvement and metastases. Histologically, ERMS has the highest 10-year survival across all age groups, whereas pleomorphic RMS has the worst outcome.2 Specifically regarding the primary site of disease, tumours in the limbs and extremity have been deemed unfavourable according to paediatric staging criteria.11 Whilst the aggressive nature of our case may be attributed to his primary site, the histological subtype diagnosed in him has the highest survival rates. Early diagnosis and specialist referral is associated with a favourable prognosis in children with RMS.4 Our patient presented to us from primary care 1 year after his initial symptoms. This underscores that socio-economic barriers and primary care vigilance remain challenges in the South African context.
Our case of ERMS with small bowel metastases provides a valuable learning point for clinicians encountering patients with a suspected non-benign extremity mass and nonspecific abdominal complaints.
Conflict of interest
The authors declare no conflict of interest.
Funding source
No funding was required.
Ethical approval
The author/s declare that this submission is in accordance with the principles laid down by the Responsible Research Publication Position Statements as developed at the 2nd World Conference on Research Integrity in Singapore, 2010. Written consent obtained by the guardians of the patient as included in submission.
ORCID
P Joubert https://orcid.org/0000-0001-5632-722X
M Mihalik https://orcid.org/0000-0002-5852-6457
REFERENCES
1. Parham DM, Barr FG. Classification of rhabdomyosarcoma and its molecular basis. Adv Anat Pathol. 2013;20:387-97. https://doi.org/10.1097/PAP.0b013e3182a92d0d. [ Links ]
2. Amer KM, Thomson JE, Congiusta D, et al. Epidemiology, incidence, and survival ofrhabdomyosarcoma subtypes: SEER and ICES database analysis. J Orthop Res. 2019;37(10):2226- 30. https://doi.org/10.1002/jor.24387. [ Links ]
3. Dasgupta R, Rodeberg DA. Update on rhabdomyosarcoma. Semin Pediatr Surg. 2012;21(1):68-78. https://doi.org/10.1053/j.sempedsurg.2011.10.007. [ Links ]
4. Egas-Bejar D, Huh W. Rhabdomyosarcoma in adolescent and young adult patients: current perspectives. Adolesc Health Med Ther. 2014;5(1):115-25. https://doi.org/10.2147/AHMT.S44582. [ Links ]
5. Dagher R, Helman L. Rhabdomyosarcoma: an overview. Pediatr Oncol. 1999;4:34-44. https://doi.org/10.1634/theoncologist.4-1-34. [ Links ]
6. Van der Graaf WTA, Orbach D, Judson IR, Ferrari A. Soft tissue sarcomas in adolescents and young adults: a comparison with their paediatric and adult counterparts. Lancet Oncol. 2017;18(3):166-75. https://doi.org/10.1016/S1470-2045(17)30099-2. [ Links ]
7. Chen J, Liu X, Lan J, et al. Rhabdomyosarcoma in adults: case series and literature review. Int J Womens Health. 2022;14:405-14. https://doi.org/10.2147/IJWH.S352143. [ Links ]
8. Van Gaal JC, De Bont ESJM, Kaal SEJ, Versleijen-jonkers Y, van der Graaf WTA. Building the bridge between rhabdomyosarcoma in children, adolescents and young adults: the road ahead. Crit Rev Oncol Hematol. 2012;82(3):259-79. https://doi.org/10.1016/j.critrevonc.2011.06.005. [ Links ]
9. Kim J, Yoon H, Koh K, et al. Factors of distant metastasis. Am J Roentenology. 2017;209:409-16. https://doi.org/10.2214/AJR.16.17466. [ Links ]
10. Sun KK, Shen XJ. Small bowel metastasis from pulmonary rhabdomyosarcoma causing intussusception: a case report. BMC Gastroenterol. 2019;19(71):1-4. https://doi.org/10.1186/s12876-019-0990-4. [ Links ]
11. Sultan I, Qaddoumi I, Yaser S, Rodriguez-Galindo C, Ferrari A. Comparing adult and pediatric rhabdomyosarcoma in the surveillance, epidemiology and end results programme, 1973 to 2005: an analysis of 2 600 patients. J Clin Oncol. 2009;27(20):3391-7. https://doi.org/10.1200/JCO.2008.19.7483. [ Links ]
 Correspondence:
Correspondence:
email: pierrejoub@gmail.com














