Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Surgery
versión On-line ISSN 2078-5151
versión impresa ISSN 0038-2361
S. Afr. j. surg. vol.61 no.4 Cape Town 2023
http://dx.doi.org/10.36303/sajs.3985
SURGICAL ONCOLOGY
Technical success of endoscopic stenting for malignant gastric outlet obstruction
D TaitI; MF ScribaII; C RobinsonI; EG JonasIII; GE ChinneryII
IDepartment of Surgery, Groote Schuur Hospital, University of Cape Town, South Africa
IIUpper Gastrointestinal Surgery Unit, Department of Surgery, Groote Schuur Hospital, University of Cape Town, South Africa
IIISurgical Gastroenterology, Department of Surgery, Groote Schuur Hospital, University of Cape Town, South Africa
ABSTRACT
BACKGROUND: Palliation of irresectable malignant gastric outlet obstruction (GOO) using self-expanding metal stents (SEMS) is gaining popularity with high technical success rates. The aim of this study was to review and compare GOO stenting for malignancy with other series
METHODS: A retrospective review of all patients undergoing pyloroduodenal stenting for malignant GOO at Groote Schuur Hospital, 1 March 2018-31 August 2021, evaluating demographics, technical success, pathology, and stent-related complications was done
RESULTS: One hundred and fourteen patients, of which 38.6% were female, were included, with gastric malignancies (74.6%) being the most frequent underlying pathology. Median age was 64 years (IQR 53-70 years), with 48.2% having at least one comorbidity. The majority (96 patients; 85.7%) required only one stent. In total, 132 stent insertion attempts were undertaken. Three technical failures were experienced (one incorrect stent placement and two failed insertions), equating to a 97.4% technical success rate. Four immediate complications occurred (3.1%): two related to sedation, one incorrect stent placement and an oesophagogastric junction perforation with procedural death. Fifteen delayed complications occurred: 13 tumour in-growth blockages, one stent fracture and one case of poor radial stent expansion. Stent blockages occurred at a median of 107 days (IQR 80-275 days). Salvage stenting was 100% successful in 14 cases requiring re-stenting
CONCLUSION: Technical insertion success rates of primary and salvage duodenal stenting for malignant GOO are on par with international high-volume units. The leading pathology locally is gastric adenocarcinoma, with palliative stenting remaining a feasible and accessible option.
Keywords: malignant gastric outlet obstruction, self-expanding metal stents, endoscopic stenting, duodenal stent, gastric stent
Introduction
Most gastric outlet obstruction (GOO) cases (50-80%) presenting to endoscopy units today are due to malignant obstruction.1 While gastric and pancreatic malignancies are the most common causes of malignant GOO, lymphomas, duodenal, biliary tract, ampullary and metastatic malignancies may all cause GOO.2 Although the overall incidence of gastric cancer appears to be declining in the western hemisphere, the proportion it now contributes to the aetiology of GOO has increased since benign peptic ulcer disease (PUD) as primary aetiology has markedly decreased.3
For patients with irresectable malignant disease and a short life expectancy, placement of a permanent self-expanding metal stent (SEMS) via endoscopy with fluoroscopic guidance is safe, effective, and well-established. The goal of stent placement is to relieve the obstructive symptoms and to improve early oral intake, thereby improving quality of life and avoiding the potential associated morbidity of surgery and anaesthesia.4 Compared to surgical gastrojejunostomy, endoscopic stenting has an earlier return to oral intake and a shorter hospital stay.5,6 While the cost of these stents can be considerable, the endoscopic placement of a gastroduodenal stent is more cost effective when compared to surgery.7,8
This is mainly due to reduced post-procedural length of hospital stay and avoiding postoperative intensive care unit (ICU) admissions. In addition, patients with recurrent obstructions due to disease progression may benefit from salvage endoscopic stenting, with similar benefits.7 A more recent addition to the management options is endoscopic ultrasound (EUS)-guided gastroenterostomy using a lumen apposing metal stent (LAMS). Although early data on this technique is promising,9 not all endoscopy units are offering this service routinely.
Generally, technical success rates of endoscopic stenting are high and refer to the successful endoscopic placement and deployment of the stent across the stricture or obstruction. A complicated or significantly altered anatomy with acute angulation or severe stenosis may contribute to technical failure of guidewire passage through the stricture or stent deployment across the obstruction. Technical success in duodenal strictures can be more complicated than distal gastric obstructions, owing to the curved configuration of the duodenum, as well as loop formation of the stent delivery system in a large, distended stomach.10 In addition, due to late presentation, atonic, chronically distended and elongated stomachs filled with residual food have the added difficulties of poor visibility and challenges in reaching the area of obstruction.11
Early complications related to the endoscopic procedure include bleeding, abdominal pain, perforation, or incorrect positioning of the SEMS. As most stents are placed under conscious sedation, in the background of GOO, aspiration remains a constant concern. Late complications are invariably due to tumour overgrowth within the SEMS and rarely due to delayed SEMS migration.4 There is currently insufficient evidence regarding the preferential placement of a partially covered, covered, or uncovered SEMS.1213 The benefits of partially or fully covered SEMS are potentially longer patency rates due to minimal tumour ingrowth. However, their migration rates are considerably higher than uncovered SEMS.14 Secondary salvage SEMS placement for primary tumour ingrowth fortunately also has high technical and clinical success rates.1516 The upper gastrointestinal surgery unit at Groote Schuur Hospital is referred significant numbers of both benign and malignant GOO from a wide referral catchment area. This study reviewed SEMS placement for malignant GOO in our unit: the indications, immediate technical success, and the detection and subsequent management of early and late complications were investigated.
Methods
This is a single centre retrospective review of patients presenting to a tertiary state hospital endoscopy unit with malignant GOO requiring palliative SEMS placement. All patients presenting to the upper gastrointestinal surgery unit at Groote Schuur Hospital with clinical features of GOO between 1 March 2018 and 31 August 2021 were evaluated for potential SEMS placement. GOO was defined as a mechanical obstruction with an inability to pass a standard 10 mm gastroscope through the stricture, with clinical symptoms of GOO. Patients with histologically confirmed malignant strictures of the antropyloroduodenum deemed irresectable or with poor clinical performance status that precluded surgery were considered eligible for SEMS placement as assessed by the treating endoscopist or the oncology multidisciplinary team. (MDT) Irresectability was determined by metastatic disease or local invasion of adjacent structures on computed tomography (CT) scan. SEMS placed for benign indications or malignant proximal gastro-oesophageal obstructions were excluded.
Data were taken from an ethically approved prospective endoscopy registry (Upper Gastrointestinal Surgery Registry, HREC R031/2015). This study was approved by the University of Cape Town Human Research Ethics Committee (HREC 218/2021).
SEMS insertion technique
Endoscopic upper gastrointestinal stenting is performed in the interventional endoscopy suite using fluoroscopy, under conscious sedation with a combination of midazolam and fentanyl or propofol with the patient in the left lateral position. Through a forward-viewing scope with a minimum 3.2 mm diameter working channel, a hydrophilic soft-tipped 0.035" guidewire is advanced under fluoroscopic guidance beyond the stricture. A co-axial overtube is placed over the wire and distal intra-luminal positioning confirmed by contrast injection. An uncovered SEMS is introduced over the wire through the working channel of the endoscope and deployed under simultaneous endoscopic vision and fluoroscopy. The choice of SEMS length is at the discretion of the endoscopist but in most instances a 120 mm length SEMS is deployed (Figure 1).
Technical success was defined as correctly placing a SEMS without repositioning across the obstructing stricture at the first attempt, with confirmation of SEMS patency by contrast flow into the distal lumen.
The primary outcome was to determine the technical success of SEMS insertion for irresectable malignant GOO and compare this success rate and pathology profile in our setting to other international high-volume units.
Statistical analysis
Data exploration and analysis were done using Microsoft Excel and IBM SPSS Statistics (version 28.0.1.1). Descriptive statistics were used to characterise patient demographics, histology, technical success, and complication rates. Parametric data were reported as means with standard deviation (SD) and range, and non-parametric data were reported as median with interquartile range (IQR).
Results
During the 42-month study period, 660 upper gastrointestinal SEMS were placed for obstructive symptoms and evaluated for inclusion. Following exclusions for SEMS placements for proximal malignant upper gastrointestinal disease (489 oesophageal and oesophagogastric junction) and benign (39) indications, 132 SEMS placements were evaluated (Figure 2).
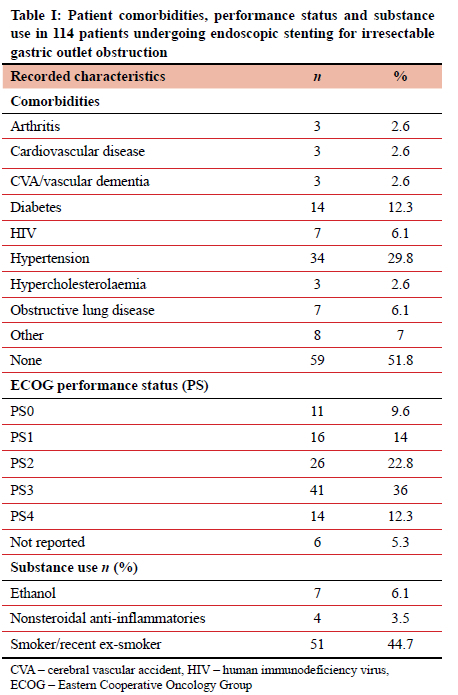
For malignant GOO, 114 patients required endoscopic stenting of the distal antrum, pylorus, or duodenum with 70 (61.4%) being male. Patients presented at a median age of 64 years (IQR 53-70). Over half (51.8%) had no documented comorbidities and 48.3% had Eastern Cooperative Oncology Group (ECOG) performance scores of 3 or 4 (Table I). Fifty-three (46.5%) patients admitted to substance use known to cause chronic irritation of the gastric mucosa, of which the most significant proportion were smokers, equating to 44.7% of this study population.
Within the cohort of 114 patients, 132 endoscopic SEMS insertion attempts were undertaken. One hundred and eighteen SEMS were used during initial, primary placement. In four patients, a single SEMS was not deemed long enough to cover the length of the stricture adequately, necessitating a second overlapping SEMS placement during the primary procedure. Three technical failures were experienced during primary placement. One SEMS was initially incorrectly placed too distally, but then immediately repositioned correctly. In the other two patients, distal enteral access with a guidewire across the malignant stricture could not be achieved. The technical success of primary SEMS placement was 97.4% with most patients (85.7%) requiring only one stent. Fourteen salvage SEMS were placed, and all were deployed successfully to lie within the previously placed SEMS.
Histopathology
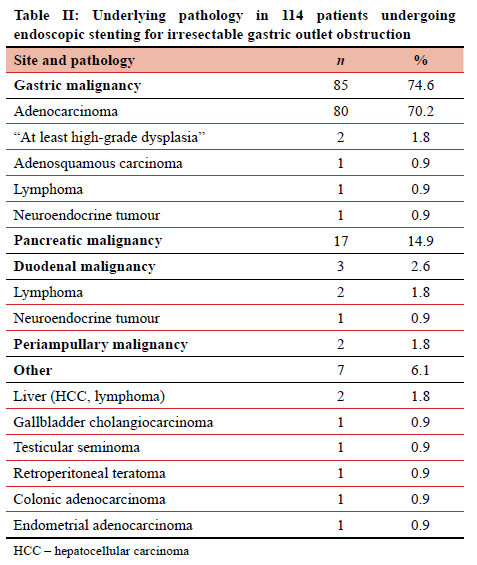
Three-quarters of the SEMS were placed for GOO caused by gastric malignancy, of which five cases were not histologically confirmed adenocarcinoma (Table II). In two patients, only high-grade dysplasia and not invasive cancer could be diagnosed. They had obvious malignant tumours on endoscopy and further imaging confirmed an irresectable malignant process. After discussion, the MDT agreed that these were likely adenocarcinoma and were then treated as such. In the 80 patients with confirmed gastric adenocarcinoma, 21 (26.3%) were noted to have diffuse-type histology. Pancreatic adenocarcinoma was the next most common, followed by duodenal and periampullary malignancies. Seven other malignancies not arising from the stomach, pancreas or duodenum necessitated duodenal stenting.
Complications
Four (3.1%) immediate insertion-related complications included two patients with sedation-related bradypnoea resulting in oxygen desaturation but successfully treated with pharmacologic sedation-reversal agents resulting in rapid improvement with no periprocedural recurrence (Table III). The single death occurred during the procedure in an elderly frail patient due to an oesophageal perforation that was only discovered on scope retraction after completion of the duodenal stenting. Before this perforation could be attended to, the patient had an unsuccessful resuscitation from a cardiac arrest.
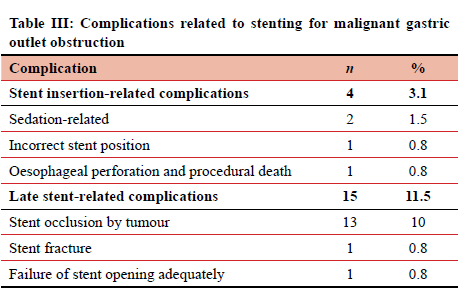
There were 15 late SEMS complications requiring repeat endoscopy. There were 13 incidents of SEMS occlusion by tumour (three in the same patient who required four SEMS). Recurrent obstructions from tumour ingrowth were successfully treated by salvage SEMS placement within the primary SEMS. The time from SEMS placement to occlusion by tumour ranged from 3 days to 548 days with a median time of 107 days (IQR 80-275 days). Early occlusions were seen with a suspected soft necrotic tumour where the uncovered SEMS caused a "cheese-wire" effect through the tumour, while later occlusion was seen from genuine tumour ingrowth.
One SEMS did not open sufficiently, requiring a second SEMS to allow for adequate gastric drainage. One asymptomatic patient had a late SEMS fracture with migration that was incidentally identified on CT scan. However, she later returned with GOO from tumour ingrowth and had a successful salvage SEMS.
Discussion
The technical success rate of gastroduodenal stenting is high. In a pooled analysis of prospective literature, which included 1 281 patients, Van Halsema et al. reported technical success rates ranging from 89.1-100%, similar to the rate of 97.4% in this study.17 The most common factors contributing to technical failure was inability to pass a guidewire across the malignant stricture, insufficient SEMS deployment and SEMS migration during deployment. While stenting of duodenal obstruction from pancreatic tumours tends to be more technically challenging,7 the technical success rate is high (up to 100%), and the rate of re-intervention low (14.3%).18 Given the local high burden of gastric malignancies, recurrent obstructions requiring reintervention did occur. In the past, SEMS in this unit were placed by using a side-viewing scope due to limitations with scope channel diameter. In this cohort, all SEMS were placed using a forward-viewing scope with a wide therapeutic channel. A front-viewing scope has the advantages of being more comfortable to use for the endoscopist and significantly shorter procedure times. While there is no difference in the technical and clinical success rates, side-viewing scopes can have a higher risk of perforation and bleeding.19
While several primary malignancies can cause GOO, advanced, irresectable gastric and pancreatic malignancies are the most prevalent. International reported literature from high-income countries has found pancreatic malignancies to be the most frequent pathology, followed by gastric cancer.41320 However, in this study, gastric adenocarcinoma dominated as the leading cause by nearly 75%. In the 2020 Global Cancer Statistics, Africa has the lowest incidence of gastric cancer worldwide, while the prevalence of pancreatic cancer is lower than in high-income countries.21 Despite gastric cancer accounting for only 1.6%22 of all reported malignancies in South Africa, most will be surgically irresectable at primary presentation. This is also confirmed by other African studies.23 The reported combination of low socio-economic status with significant diagnostic delay and resultant detrimental effects on nutrition and subsequently performance status are suspected to be similar contributing factors locally. The predominance of distal gastric adenocarcinoma with the antral/pyloric location of the tumour is a known risk factor for rapid occlusion and subsequent mortality.23 Obstructive symptoms due to diffuse-type gastric adenocarcinoma are problematic. While there may be a dominant stricture, these tumours often involve a greater proportion of the stomach area due to longitudinal gastric wall infiltration with a resultant "stiff' gastric wall and very poor distal emptying capabilities. Only if a dominant distal stricture was present in the background of a diffuse-type infiltration, was a SEMS placed. Stenting of mid-body or pan-gastric strictures does not result in significant clinical improvement due to the associated gastroparesis. Such patients represent difficult management cases and may in rare cases be amenable to palliative gastrectomy but are often referred to the oncology or palliative care team for supportive care.
Although we use exclusively uncovered SEMS in malignant GOO, there are currently many different SEMS available, that can broadly be divided into covered (CSEMS) and uncovered (UCSEMS) stents. In the American Society for Gastrointestinal Endoscopy (ASGE) Guideline of 2021, the panel found insufficient evidence in the literature to recommend one SEMS over the other.24 A large, randomised controlled trial by Yamao et al. of 366 patients compared the use of a CSEMS vs USEMS and found no difference between immediate technical success, clinical success, adverse events, and overall patient survival.12 There was a significant difference regarding late stent complications, with a much higher rate of stent migration in the CSEMS group, but more tumour ingrowth in the UCSEMS group. Since it is technically easier to place a salvage stent through a previous stent with tumour ingrowth than it would be to reposition or retrieve a migrated stent, we prefer in our setting to exclusively use UCSEMS for malignant GOO. When these late stent complications do occur and the stent must be retrieved or another salvage stent needs to be placed through the old stent, technical success rates are high. Locally our salvage stent placement has a 100% technical success rate, which falls within the 92-100% range reported in the literature.25,26 Length is another factor to consider during SEMS selection. Care must be taken when stenting specifically the second part of the duodenum. Here we recommend a SEMS long enough to be deployed across the stricture but to include an approximate 2 cm overlap across the pyloric inlet into the antrum. This is to avoid the inadvertent obstruction or duodenal side-wall erosion that may occur with straightening of the SEMS within the duodenal c-loop.
Cost is always an essential factor in resource-constrained countries such as South Africa, as is the limited availability of theatre, hospital, and ICU beds. Several studies calculating cost-effectiveness concluded that the cost of palliation with endoscopically placed SEMS was much less than with surgical gastro-jejunostomy (GJ) and allowed patients to go home earlier.7,8 The vast majority of patients required one SEMS; salvage stenting was required in 12 mainly due to subsequent tumour ingrowth. These were placed for the most part as out-patient procedures, having been referred with recurrence of GOO symptoms. There are advantagesto surgical GJ, including lower re-obstruction rates and, therefore, less re-interventions, with comparable clinical and technical success. Surgical GJ should be considered in patients with a better performance score and life expectancy, even if it means a longer hospital stay and cost.27 Many of our patients referred for stenting have advanced disease with a poor performance score, so the advantages of avoiding an anaesthetic, the morbidity of surgery, ICU admission, plus a quick return to oral intake and early discharge home are clear. Like most low- to middle-income countries (LMICs), the study site functions in a resource-constrained environment, with distinct financial benefits to early discharge post endoscopic stenting.
In 2012, Binmoeller and Shah described a novel EUS technique to create a gastro-jejunal anastomosis.9 Since then, various methods have been developed to perform an EUS-guided gastroenterostomy (EUS-GE) using LAMS.4
While the procedure is more technical to perform, more time consuming and requires skilled endoscopists, it has the advantage of avoiding open surgery with the associated morbidity and mortality risks. Compared to the more traditional placement of a SEMS, EUS-GE has shown lower rates of stent failure due to tumour ingrowth, stent migration or other obstruction, thus longer patency rates and less need for re-intervention.27 This is likely due to the stenting occurring away from the primary tumour site. In a systematic review and meta-analysis by Iqbal et al. of 285 patients,
EUS-GE had a pooled technical success of 92% (95% CI
88-95%) and clinical success of 90% (95% CI 85-94%).29 Adverse events can include stent mis-deployment, bleeding, pneumo- or haemoperitoneum, leakage, abdominal pain and peritonitis (95% CI 0-26%).30 Although the expertise for LAMS insertion is available locally and frequently used for biliary drainage and pancreatic collections, in the setting of GOO management, experience is limited in our unit.
Study limitations
Most patients referred to the study site are often from outlying regions and district hospitals. The clear limitations of our study are that we are unable to comment on the degree of subsequent improvement of GOO symptoms or duration of clinical success, or on the length of survival post palliative stenting. We remain dependent on the primary clinicians to refer patients back to us for re-intervention, should they suspect any late stent-related complications amenable to correction. We have no data on quality-of-life (QoL) post-stenting and it is not clear locally whether SEMS placement or surgical gastroenterostomy allows for better gastric drainage and subsequent greater improvement in QoL. Although we believe primary stenting to be more cost effective than surgical gastroenterostomy, the cost of subsequent interventions, specifically salvage stenting increases cost and we have no data to compare this to surgical options. However, in view of limited access to hospital beds and theatre, we still opt to attempt to re-stent those palliative patients presenting with recurrent GOO symptoms when feasible.
Conclusion
Technical success rates of both primary and salvage endoscopic SEMS placement for malignant distal gastric and duodenal obstructions in this study site are high and compare well to other series. We have a high burden of gastric carcinoma, with many patients having advanced irresectable disease or poor performance scores. Until screening and earlier detection rates improve locally, palliation of gastric cancer remains the most frequent approach for this pathology.
Local palliative endoscopic stenting of distal gastric and duodenal obstructions remains a very useful and effective non-surgical option for the relief of GOO symptoms in a LMIC setting with resource constraints and limitations.
Conflict of interest
The authors declare no conflict of interest.
Funding source
No external funding was received for this study.
Ethical approval
This study was formally reviewed and approved by the University of Cape Town Research Ethics Committee (HREC 218/2021).
ORCID
D Tait https://orcid.org/0000-0003-0877-9066
MF Scriba https://orcid.org/0000-0001-8903-0510
C Robinson https://orcid.org/0000-0002-9385-2709
EG Jonas https://orcid.org/0000-0003-0123-256X
GE Chinnery https://orcid.org/0000-0002-9097-8648
References
1. Tringali A, Giannetti A, Adler DG. Endoscopic management of gastric outlet obstruction disease. Ann Gastroenterol. 2019;32(4):330-7. https://doi.org/10.20524/aog.2019.0390. [ Links ]
2. Jeong SJ, Lee J. Management of gastric outlet obstruction: Focusing on endoscopic approach. World J Gastrointest Pharmacol Ther. 2020;11(2):8-16. https://doi.org/10.4292/wjgpt.v11.i2.8. [ Links ]
3. Samad A, Khanzada TW, Shoukat I. Gastric outlet obstruction: change in etiology. Pak J Surg. 2007;23(1):29-32. [ Links ]
4. Troncone E, Fugazza A, Cappello A, et al. Malignant gastric outlet obstruction: which is the best therapeutic option? World J Gastroenterol. 2020;26(16):1847-60. https://doi.org/10.3748/wjg.v26.i16.1847. [ Links ]
5. Hong J, Chen Y, Li J, et al. Comparison of gastrojejunostomy to endoscopic stenting for gastric outlet obstruction: an updated systematic review and meta-analysis. Am J Surg. 2022;223(6):1067-78. https://doi.org/10.1016/j.amjsurg.2021.10.038. [ Links ]
6. Upchurch E, Ragusa M, Cirocchi R. Stent placement versus surgical palliation for adults with malignant gastric outlet obstruction. Cochrane Database Syst Rev. 2018;30;5(5):CD012506. https://doi.org/10.1002/14651858.CD012506.pub2. [ Links ]
7. Fisher AV, Hanlon B, Fernandes-Taylor S, et al. Natural history and cost analysis of surgical bypass versus endoscopic stenting for the palliative management of malignant gastric outlet obstruction. HPB (Oxford). 2020;22(4):529-36. https://doi.org/10.1016/j.hpb.2019.08.009. [ Links ]
8. Johnsson E, Thune A, Liedman B. Palliation of malignant gastroduodenal obstruction with open surgical bypass or endoscopic stenting: clinical outcome and health economic evaluation. World J Surg. 2004;28(8):812-7. https://doi.org/10.1007/s00268-004-7329-0. [ Links ]
9. Binmoeller KF, Shah JN. Endoscopic ultrasound-guided gastroenterostomy using novel tools designed for transluminal therapy: a porcine study. Endoscopy. 2012;44(5):499-503. https://doi.org/10.1055/s-0032-1309382. [ Links ]
10. Ye B-W, Lee K-C, Hou M-C. Endoscopic management of malignant gastric outlet obstruction. J Chin Med Assoc. 2021;84(4):346-53. https://doi.org/10.1097/JCMA.0000000000000502. [ Links ]
11. Lopera JE, Brazzini A, Gonzalez A, Castaneda-Zuniga WR. Gastroduodenal stent placement: current status. Radiographics. 2004;24(6):1561-73. https://doi.org/10.1148/rg.246045033. [ Links ]
12. Oh D, Lee SS, Song TJ, et al. Efficacy and safety of a partially covered duodenal stent for malignant gastroduodenal obstruction: a pilot study. Gastrointest Endosc. 2015;82(1):32-6.e1. https://doi.org/10.1016/j.gie.2014.11.039. [ Links ]
13. Yamao K, Kitano M, Chiba Y, et al. Endoscopic placement of covered versus uncovered self-expandable metal stents for palliation of malignant gastric outlet obstruction. Gut. 2021;70:1244-52. https://doi.org/10.1136/gutjnl-2020-320775. [ Links ]
14. Tringali A, Costa D, Anderloni A, et al. Covered versus uncovered metal stents for malignant gastric outlet obstruction: a systematic review and meta-analysis. Gastrointest Endosc. 2020;92(6):1153-63.e9. https://doi.org/10.1016/j.gie.2020.06.033. [ Links ]
15. Jin EH, Kim SG, Seo JY, et al. Clinical outcomes of re-stenting in patients with stent malfunction in malignant gastric outlet obstruction. Surg Endosc. 2016;30(4):1372-9. https://doi.org/10.1007/s00464-015-4338-z. [ Links ]
16. Endo S, Takiguchi S, Miyazaki Y, et al. Efficacy of endoscopic gastroduodenal stenting for gastric outlet obstruction due to unresectable advanced gastric cancer: a prospective multicenter study. J Surg Oncol. 2014;109(3):208-12. https://doi.org/10.1002/jso.23486. [ Links ]
17. Van Halsema EE, Rauws EAJ, Fockens P, Van Hooft JE. Self-expandable metal stents for malignant gastric outlet obstruction: a pooled analysis of prospective literature. World J Gastroenterol. 2015;21(43):12468-81. https://doi.org/10.3748/wjg.v21.i43.12468. [ Links ]
18. Orr J, Lockwood R, Gamboa A, et al. Enteral stents for malignant gastric outlet obstruction: low reintervention rates for obstruction due to pancreatic adenocarcinoma versus other etiologies. J Gastrointest Surg. 2020;25(3):720-7. https://doi.org/10.1007/s11605-019-04512-6. [ Links ]
19. Kumar V, Ghoshal UC, Mohindra S, Saraswat VA. Palliation of malignant gastroduodenal obstruction with self-expandable metal stent using side- and forward-viewing endoscope: Feasibility and outcome. JGH Open. 2019;3(1):65-70. https://doi.org/10.1002/jgh3.12110. [ Links ]
20. Tringali A, Didden P, Repici A, et al. Endoscopic treatment of malignant gastric and duodenal strictures: a prospective, multicenter study. Gastrointest Endosc. 2014;79(1):66-75. https://doi.org/10.1016/j.gie.2013.06.032. [ Links ]
21. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-49. https://doi.org/10.3322/caac.21660. [ Links ]
22. Herbst MC. Fact sheet on stomach cancer [Internet]. Cancer Association of South Africa. Updated May 2021. Available from: https://cansa.org.za/files/2021/05/Fact-Sheet-on-Stomach-Cancer-NCR-2017-web-2021.pdf. [ Links ]
23. Bang GA, Savom EP, Oumarou BN, et al. Clinical epidemiology and mortality risk factors of gastric cancer in a sub-Saharan African setting: A retrospective analysis of 120 cases in Yaoundé (Cameroon). PanAfr Medical J. 2020;37:104. https://doi.org/10.11604/pamj.2020.37.104.25422. [ Links ]
24. Jue TL, Storm AC, Naveed M, et al. ASGE guideline on the role of endoscopy in the management of benign and malignant gastroduodenal obstruction. Gastrointest Endosc. 2021;93(2):309-22.e4. https://doi.org/10.1016/j.gie.2020.07.063. [ Links ]
25. Kim CG, Choi IJ, Lee JY, et al. Outcomes of second self-expandable metallic stent insertion for malignant gastric outlet obstruction. Surg Endosc. 2013;28(1):281-8. https://doi.org/10.1007/s00464-013-3185-z. [ Links ]
26. Mo JW, Kim YM, Kim J-H, et al. Clinical outcomes after multiple self-expandable metallic stent placement using stent-in-stent technique for malignant gastric outlet obstruction. Medicine (Baltimore). 2020;99(21):e19432. https://doi.org/10.1097/MD.0000000000019432. [ Links ]
27. Jang S, Stevens T, Lopez R, Bhatt A, Vargo JJ. Superiority of gastrojejunostomy over endoscopic stenting for palliation of malignant gastric outlet obstruction. Clin Gastroenterol Hepatol. 2019;17(7):1295-302.e1. https://doi.org/10.1016/j.cgh.2018.10.042. [ Links ]
28. Ge PS, Young JY, Dong W, Thompson CC. EUS-guided gastroenterostomy versus enteral stent placement for palliation of malignant gastric outlet obstruction. Surg Endosc. 2019;33(10):3404-11. https://doi.org/10.1007/s00464-018-06636-3. [ Links ]
29. Iqbal U, Khara H, Hu Y, et al. EUS-guided gastroenterostomy for the management of gastric outlet obstruction: a systematic review and meta-analysis. Endoscopic Ultrasound. 2020;9(1):16-23. https://doi.org/10.4103/eus.eus_70_19. [ Links ]
30. Stefanovic S, Draganov PV, Yang D. Endoscopic ultrasound guided gastrojejunostomy for gastric outlet obstruction. World J Gastrointest Surg. 2021;13(7):620-32. https://doi.org/10.4240/wjgs.v13.i7.620. [ Links ]
 Correspondence:
Correspondence:
email: galya.chinnery@uct.ac.za














