Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Surgery
versión On-line ISSN 2078-5151
versión impresa ISSN 0038-2361
S. Afr. j. surg. vol.61 no.4 Cape Town 2023
http://dx.doi.org/10.36303/sajs.4036
SURGICAL ONCOLOGY
The incidence and management of complications following stenting of oesophageal malignancies
G TeyangesikayiI; MF ScribaII; S VirannaIII; EG JonasIV; GE ChinneryIV
IDepartment of Surgery, Groote Schuur Hospital, University of Cape Town, South Africa
IIUpper Gastrointestinal Surgery Unit, Department of Surgery, Groote Schuur Hospital, University of Cape Town, South Africa
IIIDepartment of Radiation Oncology, Groote Schuur Hospital, University of Cape Town
IVSurgical Gastroenterology, Department of Surgery, Groote Schuur Hospital, University of Cape Town
ABSTRACT
BACKGROUND: Oesophageal stenting effectively palliates malignant dysphagia with reported high technical and clinical success rates approaching 90% and a low, though often problematic, complication frequency. This study aimed to benchmark success rates, the incidence and management of complications at a tertiary interventional endoscopy centre.
METHODS: This single centre three-year (March 2018-March 2021) study reviewed demographics, tumour histology/ position, and early and late complications of palliative oesophageal stenting. A multivariate analysis of tumour position association with complications was performed.
RESULTS: A total of 297 patients (73.4% squamous cell carcinoma) underwent 354 stent insertion attempts. Immediate technical insertion success rate was 97.5% with dysphagia improvement achieved in all successful insertions (100% clinical success rate). Three hundred and forty-six (98.6%) were fully covered stents, with 17 (4.8%) placed for trachea-oesophageal fistulae. Twenty-one (6.0%) immediate insertion-related complications occurred, including two oesophageal perforations, but no insertion-related mortalities. Late complications occurred in 73 (20.8%) with tumour overgrowth (10.1%) and stent migration (6.1%) being the most frequent. Of all 354 stents, 75.2% had no documented complications for the lifetime of that stent, while 68 complications required re-intervention, equating to a re-intervention rate of 19.4% per stent insertion. Stent migration was significantly higher in distal tumours (11.8% vs 1.8%, p < 0.001), while discomfort necessitating same-day stent removal was higher in proximal tumours starting at < 20 cm from the incisors (16.7% vs 0.5%, p < 0.001)
CONCLUSION: Oesophageal stenting for malignant dysphagia is peri-procedurally safe and effective. Outcomes reported from this South African cohort compare favourably to high-volume international units.
Keywords: oesophageal cancer, self-expanding metal stents, endoscopic stenting, oesophageal stent.
Introduction
Oesophageal carcinoma is the eighth most common cancer and the sixth most frequent cause of cancer-related death worldwide, with developing nations making up more than 80% of total cases and deaths.1 The highest incidence rates are found in Asia and sub-Saharan Africa, and South Africa is amongst the countries with the highest incidence rates globally.2,3 Over 50% of patients with oesophageal cancer present late with advanced, incurable or inoperable disease. In South Africa, well over 90% of patients with squamous cell oesophageal cancer present with high-grade dysphagia at primary presentation.4
While the mainstay of curative therapy remains surgery with perioperative chemoradiotherapy, stenting provides palliation of malignant dysphagia in oesophageal or proximal gastric cancer in non-surgical candidates due to extensive local or metastatic disease or poor performance status.5-7
The use of stenting has been shown to provide an effective method of palliation for malignant dysphagia. Most published series have shown overall immediate technical success rate in up to 100%, with clinical success (improvement in dysphagia score) approaching 90%. The resumption of oral intake enhances quality of life as well as the nutritional status of the patient.5-7
Advances in stent design, from the rigid plastic endoprostheses of the early 90s to the current self-expanding metal, plastic and biodegradable options, have resulted in significantly improved outcomes. However, stenting is still attendant with numerous and significant complications. Complications of stent placement can be classified as early within 2-4 weeks or delayed if occurring after this period. Early complications may occur immediately or after stent placement and include foreign body sensation, pain, gastroesophageal reflux, migration, bleeding, and perforation and have decreased in frequency because of recent advances in stent design and delivery. Late complications are more frequent and include delayed migration, tumour ingrowth and overgrowth, proximal peptic strictures, and aero-oesophageal fistula.6 Delayed complications still occur in up to 65% patients, with re-intervention rates as high as 50%.5,6 Among both early and delayed complications, migration is the most common complication, occurring with a very variable, rate ranging from 7-75% depending on stent type and design.5-7
Against this background, in patients with foregut malignancies who underwent palliative oesophageal or oesophagogastric stent placement, this study aimed to document the technical and clinical success rates and the frequency and management of complications.
Methods
This was a single centre retrospective review of patients presenting to the Groote Schuur Hospital upper gastrointestinal surgery unit with malignant dysphagia requiring palliative stent placement. The unit serves a large catchment population as one of two public sector centres in the greater Cape Town area, which has an estimated population of 5.8 million8 offering interventional endoscopy. The unit also receives patients for interventional endoscopy services from other parts of the Western Cape, neighbouring provinces, and other sub-Saharan countries.
All patients presenting with clinical features of dysphagia and requiring palliative endoscopic oesophageal stenting between 1 March 2018 and 31 March 2021 were evaluated for potential inclusion. Inclusion required histological confirmation of malignancy plus irresectability on the basis of either poor performance status, presence of metastases or local tumour invasion, as assessed on computed tomography (CT). Data on patient demographics, reported substance use and dysphagia grade were collected from a prospective endoscopy registry. The degree of dysphagia was assessed by the Mellow and Pinkas9 grading system where: 0 = normal/ no dysphagia, 1 = ability to eat some solid food, 2 = ability to eat semisolids only, 3 = ability to swallow liquids only and 4 = complete dysphagia with inability to swallow saliva. Advanced dysphagia was defined as grade 3 or 4 dysphagia.
Tumour position was determined by flexible endoscopy in centimetres from the incisors at initial and every subsequent presentation for interventional endoscopy. The most proximal aspect of the tumour was used to divide the cohort into three groups: "proximal" at < 20 cm, "mid" at 20-30 cm and "distal" at > 30 cm.
Stent insertion
Oesophageal stent placement was performed using fluoroscopy in the interventional endoscopy unit with the patient under conscious sedation, using a combination of short-acting opioids and benzodiazepines. Once standard diagnostic upper gastrointestinal endoscopy has been performed and the most proximal margin of the tumour identified, passage of the scope beyond the tumour is attempted, but if not possible, contrast is injected and the fluoroscopically visible stricture measured in centimetres. At the endoscopists discretion, either a stiff metal Savary-Gilliard wire with flexible tip or a standard 0.035" Jag wire is passed across the stricture under fluoroscopic guidance, ensuring that the wire crosses the diaphragm and enters the stomach. The endoscope is then retracted, leaving the wire in situ. A stent of appropriate length is then passed over the wire and correctly positioned and deployed under fluoroscopy. In very proximal tumours, stent deployment is performed under simultaneous fluoroscopy and direct endoscopic vision. This is to ensure the proximal flange deploys below the cricopharyngeus muscle.
Fully covered stents were the preferred initial choice, with partially covered or uncovered stents reserved for cases of recurrent stent migration. Choice of stent length was at the discretion of the endoscopist and usually based on tumour length. Only distally-deploying stents were used.
Patients with distal tumours, where the deployed stent is shown to cross the oesophagogastric junction (OGJ), were routinely discharged on twice-daily proton pump inhibitor therapy. No anti-reflux stents were inserted in this cohort. Technical stent insertion success was defined as correctly inserting a stent across the obstructing stricture under endoscopic and fluoroscopic guidance with no repositioning required and with flow of contrast demonstrated through the stent into the distal lumen beyond the stricture.
All patients were observed in the post-procedural recovery room and once satisfactorily recovered were either discharged home or returned to their referral facility. Discharge follows a patient-reported improvement in swallowing from baseline. Contrast swallows are not routinely performed prior to discharge. Follow-up occurred with the oncology service or the palliative care team. These patients are not routinely followed-up in the endoscopy unit and were only seen again if they were re-referred or self-presented with stent-related complications or symptoms requiring repeat endoscopy.
The primary objective of this study was to determine the technical outcomes and complications of palliative endoscopic stenting of malignant oesophageal dysphagia. It aimed to determine the incidence and subsequent management of immediate and late complications associated with stent placement. Complications, using the number of stent insertions as the denominator, were then compared by tumour site to identify whether certain complications occurred more frequently based on the position of the tumour. Local results were compared to other high volume endoscopy units as regards patient demographics, pathology and complications rates.
Data exploration and analysis was done using Microsoft Excel and IBM SPSS Statistics (version 28.0.1.1). Patient demographics, histology, technical success, and complication rates were described using simple descriptive statistics. Parametric data were described using mean with standard deviation (SD) and non-parametric data were described using median with interquartile range (IQR). Comparison of complications by tumour site was performed using the Kruskal-Wallis test to allow for comparison of three groups with non-parametric data. Further post-hoc multiple comparison analyses using the Mann-Whitney U test were performed on any variables shown to be statistically significant. A _p-value of < 0.05 was considered statistically significant.
Results
Over the three-year study period (March 2018-March 2021) 297 patients required 354 (49 requiring multiple) oesophageal stent insertions for malignant disease. Of these patients, 143 (48.14%) were males with a mean age of 62.9 (95% CI 61.5-64.3) years (Table I). Female patients had a mean age of 64.7 (95% CI 62.8-66.6) years. No medical comorbidities were present in 129 patients (43.43%), while the remaining 168 patients had 247 separate comorbidities. Substance use, detailed in Table I, was admitted to by 133 obstructing strictures. Table II outlines the site of tumour obstruction and histology. Most adenocarcinomas were situated distally, with 57 of 63 (90.5%) tumours starting at 30 cm or more from the incisors. In eight patients, the primary oesophageal malignancy was neither SCC nor adenocarcinoma, and included four undifferentiated carcinomas, three oesophageal neuroendocrine carcinomas, and one oesophageal melanoma. A further eight patients were obstructed due to extrinsic malignant compression and included primary lung cancer (five), metastatic lymph node disease from cancer of the cervix (two) and breast cancer (one).When comparing demographics of the patients with adenocarcinoma (n = 63) versus those with SCC (n = 218), it is evident that the average ages were equal in the two groups (62.6 ± 12.2 years for adenocarcinoma and 62.9 ± 12.2 years for SCC), but that SCC patients were more likely to be female compared to those with adenocarcinoma (57.8% vs 31.7%, respectively, p = 0.0002).
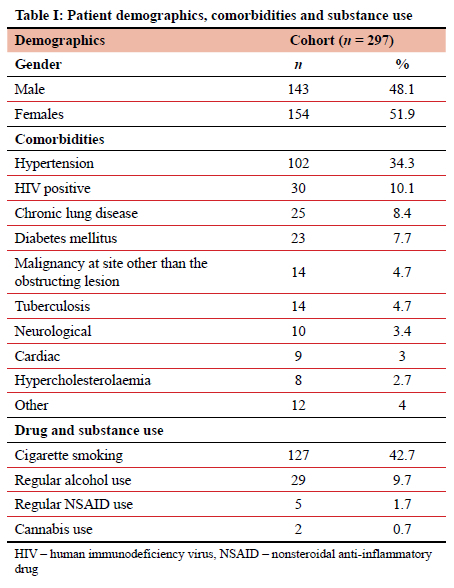
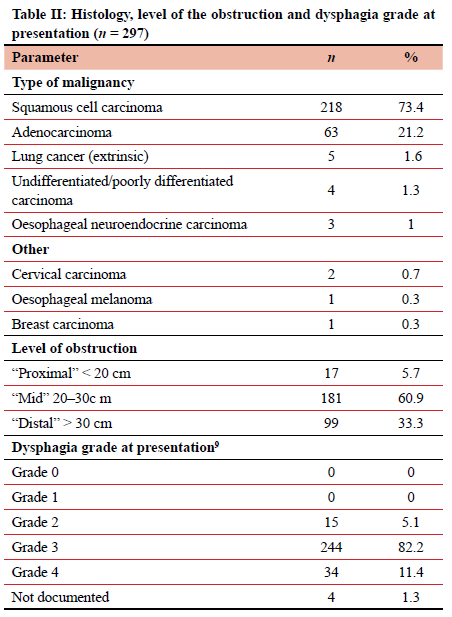
Stent insertions
A total of 354 stent insertion attempts were undertaken over the study period, with only three unsuccessful insertions and six incorrectly placed stents, which were all immediately addressed, equating to an immediate technical insertion success rate of 97.5%. Of the 351 inserted stents, 346 (98.6%) were fully covered stents, with only two partially covered stents and three uncovered stents inserted. Seventeen stents (4.8%) were placed for a confirmed trachea-oesophageal fistula. Most patients (248 patients, 83.50%) required only one stent (Table III).
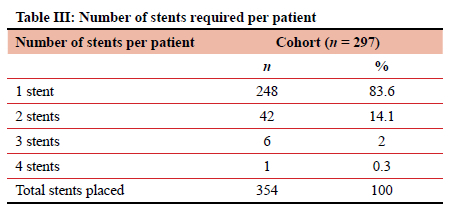
Complications
The 21 patients (6%) who had immediate insertion-related complications are detailed in Table IV Five patients required removal of the stent on the same day as they did not tolerate the stent due to high placement with globus sensation or significant associated chest pain. Two perforations caused by the primary stent insertion (and not a concomitant oesophageal dilatation) both perforated just below the cricopharyngeus, and the perforations could not be stented. During the attempted stent insertion in one patient with a very proximal lesion, the perforation was diagnosed just above the lesion. The stent was deployed to cover the perforation; however, it was immediately removed as the proximal flange opened above the cricopharyngeus. The patient was managed supportively as he had a poor performance status and was subsequently fit for a percutaneous endoscopic gastrostomy placement prior to discharge. The second proximal perforation occurred due to the wire or the stent deployment device in a patient with a distal oesophageal stricture. On this occasion, the malignant stricture was stented, but again the perforation was too proximal to allow stenting. The patient recovered after a period of conservative management and was discharged home swallowing well without further intervention. Relief of dysphagia was registered in all successful stent insertions allowing for oral intake at the time of discharge, including patients managed for acute stent-related complications (100% clinical success rate). There was no mortality due to immediate stent insertion-related complications.
Late complications occurred in 73 cases (20.8%) (Table IV). The most frequent complications requiring re-intervention were tumour overgrowth (30; 10.1%) and stent migration (18; 6.1%). Repeat intervention for tumour overgrowth occurred at a median of 63.5 days (IQR 41.0-103.3 days) after primary stenting. Stent migration occurred in 18 patients at a median 45.0 days (IQR 12.8-87.8 days) post procedure.
Of the 351 placed stents, 264 (75.2%) had no documented complications for the lifetime of that stent. Despite appropriate acid suppressive treatment, severe symptomatic volume reflux remained a significant complaint in 12 patients prompting repeat endoscopy.
Comparison of stent-related complications by tumour site group (proximal, mid and distal) is shown in Table V Immediate complications (as a group) were more likely in the proximal group (27.8% vs 5.5% for mid tumours and 5% for distal tumours, p < 0.001). When severe pain or globus sensation requiring same day stent removal were sub-analysed, these were also statistically higher in the proximal tumour group (16.7% vs 0.5% vs 0.8%, p < 0.001). In the 336 stents inserted in the mid and distal groups, only two stents required same day removal for pain or globus (0.6%). Later complications (as a group) did not differ significantly between the three groups; however, stent migration was significantly higher in the distal group compared to the mid group (11.8% vs 1.8%, p < 0.001).
Discussion
This large retrospective study describes the outcomes of palliative oesophageal stent insertions for inoperable malignancy in a tertiary-level, referral hospital endoscopy unit, over a period of three years. Most patients present very late with dysphagia, some with malignant fistulae, and others with malignancy-related haemorrhage. Self-expanding metal stents (SEMS) have become the first-choice palliative intervention in our setting owing to easy access with rapid relief of symptoms.47 Our technical success rate of 98.6% compares well with both international and other African data (95-100%).5"7·1012 All successfully stented patients had significant improvement in dysphagia grade, and/or control of tracheoesophageal fistulae allowing for oral feeds at time of discharge (a clinical success rate of 100%).
While early complications are decreasing because of recent advances in SEMS design and their delivery systems, delayed complications still occur in up to 65% patients, with a re-intervention rate as high as 50%.5,6 In most series, among both early and delayed complications, migration is the most common complication occurring at a rate of 7-75% depending on the type and design of stent.5-8,10,13 Our series has shown a similar trend with 6% immediate insertion-related complications, while late complications occurred in 20.8%. Tumour overgrowth was the most common complication and occurred more frequently than migration at 10.1% compared to 6.1% respectively.
While our outcomes are similar to those reported internationally,7,11 there is a notable variation in these outcomes. Selinger et al. report on a series of 137 patients from England with an oesophageal adenocarcinoma (AC) incidence of 57%, which is notably higher than reported in this series (21.2%).14 This is the general trend where European and American centres have higher AC rates compared to African and Asian nations.14-18 Their outcomes correlate well with our results in technical and clinical success rates. Tumour overgrowth rates are almost identical at around 10%, while migration rate in this study of 6.1% was half that reported in other studies. The higher migration rate noted in the international literature is likely explained by comparatively fewer distal cancers in our series. Our overall complication rate was 29.3% compared to the 41.6% in the report from Selinger et al.14 We suspect that our low overall complication rate is largely due to fewer stent migrations although underestimation due to the lack of dedicated follow-up may have also contributed.
In addition, we have also confirmed a local regional difference in clinical presentation within South Africa as regards the two dominant oesophageal pathologies. While an AC rate of 5.6-6.7% was reported by Loots et al. from KwaZulu-Natal, resulting in an SCC to AC ratio of 13:1 to 15.9:1, our ratio in this cohort is significantly different at 3.5:1.4,19 We suspect divergent population group proportions present in the various South African provinces with different risk profiles to play a role as regards presenting pathology. Govender et al., also from KwaZulu-Natal, report a stent migration rate of only 2.2% based on a cohort of 506 stents placed from 2007 to 2011.7 By comparison, our stent migration rates (6.1%) are more than double. We postulate that this is in part related to the differences in obstruction position, associated with our divergent pathology ratios across the provinces. Our data has shown that distally placed stents are significantly more likely to migrate, and that the AC incidence is 21.2% compared to the 5.6-6.7% reported in KwaZulu-Natal.4,19 Our distal stent positioning significantly affecting migration rates concurs with previous reports, where fully covered stents, benign conditions and distal location are variables independently associated with migration.20,21
Significant volume reflux requiring repeat endoscopy occurred in 12 of 297 patients (4%) in our series. This included only those patients who returned to the unit for repeat endoscopy, and the rate of reflux is possibly much higher as other patients may have opted not to return to hospital for this or were managed at other hospitals. Volume reflux is one of the major complications related to distal oesophageal or OGJ stent placement. A few small studies suggest a reduction in volume reflux symptoms, using antireflux stent designs, but in a recent systematic review and meta-analysis of randomised controlled trials, Pandit et al. found a trend towards reduced dysphagia with anti-reflux stents compared with standard stents, but no statistical difference with regards to volume reflux.20 Anti-reflux stents were unavailable during the study period and thus not used in this cohort. Further prospective randomised trials, ideally involving local sites, of more efficacious anti-reflux designs are required before their use can be recommended.
Study limitations
A limitation of this study is the potential under-reporting of late complications. Although the endoscopy registry used for the data collection is prospectively maintained, the retrospective analysis of complications and the fact that patients only return by self-referral or referral from other centres for symptoms means data accuracy is compromised. This lack of routine follow-up and that patients could have been managed at other institutions probably limited our ability to identify all complications.
Conclusion
Oesophageal stenting has proven to be a very effective, easily accessible, and safe method for treating palliative malignant dysphagia. Technical success, complications, and clinical outcomes in this cohort from a tertiary state hospital endoscopy unit in South Africa compare well to other highvolume endoscopy centres.
Acknowledgement
Thank you to Dr Chanel Robinson (PhD) for her assistance with the statistical analyses in this paper.
Conflict of interest
The authors declare no conflict of interest.
Funding source
No external funding was received for this study.
Ethical approval
Ethical approval for the registry (Upper Gastrointestinal Surgery Registry, HREC R031/2015) and this study (HREC 219/2020) were obtained from the Human Research Ethics Committee of the University of Cape Town.
ORCID
G Teyangesikayi https://orcid.org/0000-0002-4108-8753
MF Scriba https://orcid.org/0000-0001-8903-0510
S Viranna https://orcid.org/0000-0001-6797-1256
EG Jonas https://orcid.org/0000-0003-0123-256X
GE Chinnery https://orcid.org/0000-0002-9097-8648
REFERENCES
1. Napier KJ, Scheerer M, Misra S. Oesophageal cancer: a review of epidemiology, pathogenesis, staging workup and treatment modalities. World J Gastrointest Oncol. 2014;6(5):112-20. https://doi.org/10.4251/wjgo.v6.i5.112. [ Links ]
2. Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19(8):1893-907. https://doi.org/10.1158/1055-9965.EPI-10-0437. [ Links ]
3. Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends - an update. Cancer Epidemiol Biomarkers Prev. 2016;25(1):16-27. https://doi.org/10.1158/1055-9965.EPI-15-0578. [ Links ]
4. Loots E, Sartorius B, Madiba TE, Mulder CJJ, Clarke DL. Oesophageal squamous cell cancer in a South African tertiary hospital: a risk factor and presentation analysis. S Afr J Surg. 2017;55(3):42-46. [ Links ]
5. Kim SG, Yang CH. Upper gastrointestinal stent. Clin Endosc. 2012;45:386-91. https://doi.org/10.5946/ce.2012.45A386. [ Links ]
6. Kim KY, Tsauo J, Song HY, Kim PH, Park JH. Gastroenterology and hepatology - self-expandable metallic stent placement for the palliation of oesophageal cancer. J Korean Med Sci. 2017;32:1062-71. https://doi.org/10.3346/jkms.2017.32.7.1062. [ Links ]
7. Govender M, Aldous C, Ferndale L, Thomson SR, Clarke DL. Self-expanding metal stent placement for oesophageal cancer without fluoroscopy is safe and effective. S Afr Med J. 2015;105(10):858-61. https://doi.org/10.7196/SAMJnew8329. [ Links ]
8. 2023 World Population Review. Available from: https://worldpopulationreview.com/world-cities/cape-town-population. Accessed 8 Mar 2023. [ Links ]
9. Knyrim K, Wagner HJ, Bethge N, Keymling M, Vakil N. A controlled trial of an expansile metal stent for palliation of oesophageal obstruction due to inoperable cancer. N Engl J Med. 1993;329(18):1302-7. https://doi.org/10.1056/NEJM199310283291803. [ Links ]
10. Schoppmann SF, Langer F, Prager BG, Zacherl J. Outcome and complications of long-term self-expanding oesophageal stenting. Dis Oesophagus. 2013;26:154-8. https://doi.org/10.1111/j.1442-2050.2012.01337.x. [ Links ]
11. Burstow M, Kelly T, Panchani S, et al. Outcome of palliative oesophageal stenting for malignant dysphagia: a retrospective analysis. Dis Oesophagus. 2009;22:519-25. https://doi.org/10.1111/j.1442-2050.2009.00948.x. [ Links ]
12. Battersby NJ, Bonney GK, Subar D, et al. Outcomes following oesophageal stent insertion for palliation of malignant strictures: a large single centre series. J Surg Oncol. 2012;105:60-5. https://doi.org/10.1002/jso.22059. [ Links ]
13. Włodarczyk JR, Kuzdzal J. Stenting in palliation of unresectable oesophageal cancer. World J Surg. 2018;42:3988-96. https://doi.org/10.1007/s00268-018-4722-7. [ Links ]
14. Selinger CP, Ellul P, Smith PA, Cole NC. Oesophageal stent insertion for palliation of dysphagia in a district general hospital: experience from a case series of 137 patients. Q J Med. 2008;101:545-8. https://doi.org/10.1093/qjmed/hcn045. [ Links ]
15. Buckle G, Mahapatra R, Mwachiro M, et al. Optimal management of oesophageal cancer in Africa: a systemic review of treatment strategies. Int J Cancer. 2021;148:1115-31. https://doi.org/10.1002/ijc.33299. [ Links ]
16. Buckle G, Mrema A, Mwachiro M, et al. Treatment outcomes of oesophageal cancer in Eastern Africa: protocol of a multi-centre, prospective, observational, open cohort study. BMC Cancer. 2022;22:82. https://doi.org/10.1186/s12885-021-09124-5. [ Links ]
17. Krishnamurthy A, Behuria SS. Demographic trends in carcinoma oesophagus from India along with a brief comparative review of the global trends. South Asian J Cancer. 2020;9(3):163-7. https://doi.org/10.1055/s-0041-1726139. [ Links ]
18. Arnold M, Ferlay J, Van Berge Henegouwen MI, Soerjomataram I. Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut. 2020;69:1564-71. https://doi.org/10.1136/gutjnl-2020-321600. [ Links ]
19. Loots E, Ramdial PK, Sartorius B, Mulder CM, Clarke DL. Malignant and pre-malignant oesophageal pathology in a South African teaching hospital. S Afr J Surg. 2018;56(1):21-24. https://doi.org/10.17159/2078-5151/2018/v56n1a2076. [ Links ]
20. Pandit S, Samant H, Morris J, Alexander S J. Efficacy and safety of standard and anti-reflux self-expanding metal stent: a systematic review and meta-analysis of randomised controlled trials. World J Gastrointest Endosc. 2019;11(4):271-80. https://doi.org/10.4253/wjge.v11.i4.271. [ Links ]
21. Seven G, Irani S, Ross AS, et al. Partially versus fully covered self-expanding metal stents for benign and malignant oesophageal conditions: a single centre experience. Surg Endosc. 2013;27(6):2185-92. https://doi.org/10.1007/s00464-012-2738-x. [ Links ]
 Correspondence:
Correspondence:
email: galya.chinnery@uct.ac.za














