Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Surgery
versão On-line ISSN 2078-5151
versão impressa ISSN 0038-2361
S. Afr. j. surg. vol.61 no.4 Cape Town 2023
http://dx.doi.org/10.36303/sajs.4005
UPPER GASTROINTESTINAL SURGERY
Factors influencing outcome in patients with perforated peptic ulcer disease at a South African tertiary hospital
JJ NanackI; L FerndaleII
IDepartment of General Surgery, University of KwaZulu-Natal, South Africa
IIDepartment of Gastro-Intestinal Surgery, Grey's Hospital, South Africa
ABSTRACT
BACKGROUND: Perforated peptic ulcer (PPU) is associated with significant morbidity and mortality, particularly in low to middle income countries. This study aimed to scrutinise the clinical course of patients diagnosed with PPU and identify modifiable factors to improve outcomes.
METHODS: A retrospective review of the hybrid electronic medical record (HEMR) database at Grey's Hospital was performed. All patients diagnosed with PPU between January 2013 and December 2020 were entered into the study. The variables collected include age, ethnicity, comorbid profile, Boey score, type of surgery performed and complications. These factors were analysed to determine the factors responsible for morbidity and mortality.
RESULTS: One hundred and ninety four patients were diagnosed with PPU during the study period. Six patients were treated non-operatively, all of whom survived. In the surgically treated group, omental patch repair was performed in 159 (84.5%) patients, and primary closure in 26 (13.8%) patients. The leak rate was 32% in the cohort that underwent relaparotomy and the overall mortality was 14%. There was no significant relationship between the type of repair performed and outcome. All patients had a Boey score of 1 or more. The following factors were found to increase the probability of in-hospital mortality: age > 40 years (OR: 8.49, 95% CI 2.46-29.29 p < 0.01), female gender (OR: 2.509, CI 0.98-6.37, p = 0.048), need for relaparotomy (OR: 0.398, CI 0.17-0.91, p = 0.027) and Boey score > 1 (OR: 46.437, CI 6.13-350.28, p < 0.01). A Boey score > 1 was the only variable that increased the likelihood of finding a leaking repair at relaparotomy (p < 0.01).
CONCLUSION: The Boey score was a significant predictor of mortality and leak rate in our patients with PPU. Adding age as a variable may improve the ability to predict mortality in our setting, while the impact of gender and ethnicity needs further investigation.
Keywords: perforated peptic ulcer, omental patch, debridement and primary repair, Boey score
Introduction
Peptic ulcer disease (PUD), defined as a digestive tract injury that results in a mucosal break greater than 3-5 mm with penetration extending to the submucosal layer, has been on the decline since the advent of proton pump inhibitors and H Pylori eradication regimens. Patients who suffer from PUD can follow a variable clinical course, ranging from rapid recovery with medical management to those who develop complications of bleeding, perforation or gastric outlet obstruction.14
Perforation occurs when all the layers of the digestive tract are breached and is one of the most devastating complications of PUD, which can lead to major morbidity and death.5 Advanced age, significant comorbidities and delay in presentation are established risk factors for poor outcome in patients with perforated peptic ulcer (PPU).6,7 This is especially debilitating in countries with resource constraints.78 There is a paucity of information on the clinical course and outcomes of patients with PPU disease, especially in low and middle income countries (LMICs).9
A number of scoring systems are available in predicting the outcome of PPU disease with the Boey score, peptic ulcer perforation score (PULP), and the American Society of Anesthesiologists (ASA) score being the most commonly used.10 While the PULP score shows good predictability for mortality, it is complex and impractical in the clinical setting.11 The ASA score is not specific for PPU and is further limited by its subjective nature.12 Currently, the Boey score is one of the most widely used scoring systems for predicting outcomes in PPU due to its relatively simple nature combined with high accuracy.10,13, 14
This study aims to describe the clinical course that patients with perforated PUD follow and identify factors that contribute to poor outcomes. Insights will be used to guide management protocols of patients presenting with PPU.
Methods
A retrospective review of the hybrid electronic medical record (HEMR) database at Grey's Hospital was performed. All patients diagnosed with PPU at Grey's Hospital between January 2013 and December 2020 formed the cohort for analysis. Grey's Hospital is a tertiary hospital that provides health care to one of three major areas in KwaZulu-Natal.
The area is home to approximately three million people, two-thirds of whom reside in rural areas. The drainage area consists of 3 regional and 17 district referral hospitals.15
Patients who were 13 years and older who had a confirmed single diagnosis of perforated peptic ulcer disease were included in the study.
Data collected included patient demographics, ethnicity (which was self-declared by the patient), clinical presentation, medical comorbidities, Boey score, management, and outcome. Outcome variables collected included in-hospital mortality, leak rate, need for relaparotomy and ICU stay. Comorbidities that were documented included hypertension, diabetes mellitus, human immunodeficiency virus (HIV) infection status, and coronary artery disease. The use of non-steroidal anti-inflammatory drugs was included as a comorbidity due to its association with PUD.
The Boey score was calculated based on three factors: delayed presentation more than 24 hours, systolic blood pressure less than 100 mmHg and the prescence of a major comorbidity. The patient was given one point for each of the three factors that were present; the minimum score obtainable was zero while the maximum was 3.13
Operations performed included omental patch repair, debridement with primary repair, partial gastrectomy, and repair over a t-tube. All operations were performed through a midline laparotomy incision. For patients who underwent relaparotomy, a note was made of whether the initial repair was intact or leaking.
The decision to treat patients non-operatively was based on clinical findings as well as imaging in the form of computer tomography (CT) scans or contrast meals confirming a contained leak. They were managed conservatively with helicobacter pylori eradication therapy and discharged once they were tolerating a full ward diet and the abdominal pain had resolved.
Ulcer location was divided into 3 categories based on the anatomical description of the location of the ulcer as described in the operative notes. This included gastric, pre-pyloric, and duodenal. Gastric ulcers included all those found in the fundus, body or antrum of the stomach. Pre-pyloric ulcers included all ulcers found within 5 cm of the pylorus, and duodenal ulcers included all those found distal to the pylorus up to the fourth part of the duodenum.
Length of stay in hospital was expressed as days from admission to discharge.
Statistical analysis
Graphical and descriptive statistics were used to present the clinical characteristics. The Kolmogorov-Smirnov test was used to determine a normal distribution or not. With respect to characteristics of patients with the prevalence of PPU we used the Mann-Whitney U test to check for significant differences in the ethnicity. We also used the Kruskal-Wallis test to check for differences in ethnicity with respect to the characteristics of patients who presented with ulcer complications.
The chi-square test of independence and the robust chi-square test of independence were used to test the relationship between factors and outcomes, since there was a need to assess the influence of variables such as age on the survival outcome or ethnicity and its relation to the prevalence of PPU. The Boey score and its effect on the length of stay in hospital and death was tested using the eta squared statistic and the one-way analysis of variance (ANOVA).
Results
One hundred and ninety four patients were included in the cohort. Table I shows the demographic profile, comorbidities and deaths in the cohort. The male-to-female ratio was 5:1. The mean age was 45 years, and three quarters of the patients were of African ancestry. Hypertension was the most common comorbidity, and non-steroidal anti-inflammatory the only PUD risk factor recorded.
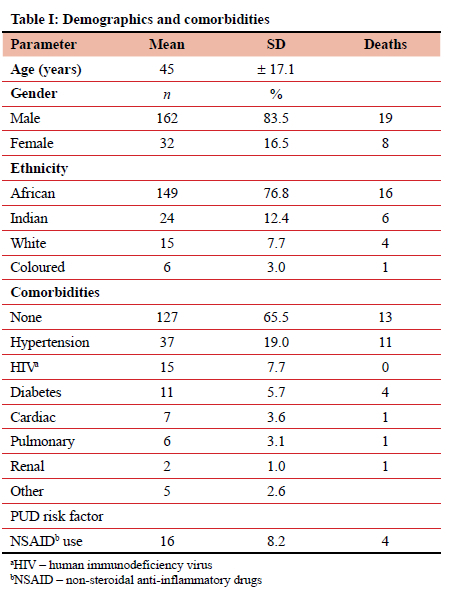
Of the 11 individuals who died and possessed the risk factor of hypertension, only 3 had exclusive hypertensive disease as a comorbidity, whereas the rest were associated with other comorbidities, most commonly diabetes. Two of the patients who died had NSAID use as their only risk factor for PPU disease. One case of documented coronary artery disease was present in one of the patients who died, along with one individual who had documented hypertension and renal disease.
The most common ulcer location was pre-pyloric in 59.3% of the study population, followed by duodenal in 21.6% and gastric in 18.6%.
Figure 1 details the management and outcome of the patients in our cohort. Omental patch repair was the most common operative procedure in 84.5% of surgically treated patients, followed by primary closure in 13.8%. Two patients underwent partial gastrectomy and one had a repair over a t-tube.
Seventy-five of the 188 individuals who underwent a laparotomy required a relaparotomy and of those, 51 revealed an intact repair, resulting in a confirmed leak rate of 32%.
The overall mortality was 14%. African patients had a lower mortality compared to the other races (p = 0.02).
The mortality for females was significantly higher than for males: 25% vs. 12% (p = 0.04).
Of the patients included in this study who did not require relaparotomy, 84% had an omental patch at their index operation, and 14% had debridement and primary closure at their index surgery. There was no significant relationship between the type of repair performed and operative outcome. None of the patients had a Boey score of 0 (Table II).
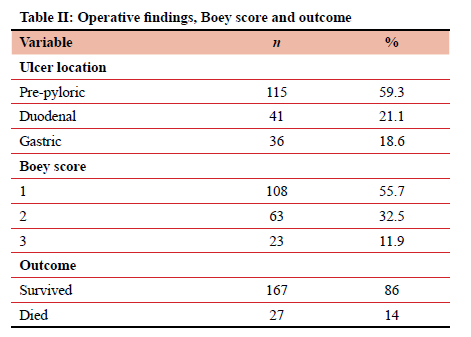
Table III shows the significant factors that increased the probability of in-hospital mortality on multivariate analysis. These factors were age > 40 years (OR: 8.49, 95% CI 2.4629.29 p < 0.01), female gender (OR: 2.509, CI 0.98-6.37, p = 0.048), need for relaparotomy (OR: 0.398, CI 0.17-0.91, p = 0.027) and Boey score > 1 (OR: 46.437, CI 6.13-350.28, p < 0.01). The only variable that significantly increased the chances of finding a leaking repair at relaparotomy on multivariate analysis was a Boey score > 1 (likelihood ratio: 0.05, p-value: 0.006).
Discussion
In this study, we have found in patients with PPU that age, female gender, ethnicity, need for relaparotomy and Boey score were factors that significantly increased the probability of in-hospital mortality. The mortality rate of 13.9% in this study population was higher than the 10% reported in international literature.161, 7 This could be explained by the fact that most of the patients had a Boey score of 1 and none had a score of 0. In contrast, data from international literature indicates that the majority of patients have a Boey score of 0 at presentation.
The mean age of 45 and the male-to-female ratio of 5:1 corresponds to the demographic profile of international cohorts.6 Age greater than forty, white race group, female gender, and the need for relaparotomy are not modifiable factors. The association between increased age and mortality is consistent with other studies.18 The increased risk associated with female gender has not been validated by data from international literature.19 One possible explanation for this finding is that the female patients in our cohort were older than the male patients (58.44 years vs. 42.48 years). To our knowledge, this is the first study to show the effect of ethnicity on mortality in PPU, and further investigation is needed to confirm or refute the association.
The Boey score was the only independent predictor of a leak. The mean Boey score of patients who had a leak was 2, and may relate to the fact that all the parameters in the score promote or reflect physiological derangement that could contribute to poor healing.
The omental patch was the most popular operative technique employed in the surgical management of the patients in this study population due to the surrounding tissue being too friable for primary closure. This technique displayed favourable results with respect to relaparotomy findings, with 70.3% revealing intact repairs.
The indications for gastrectomy in two of the patients were based on the decision of the operating surgeon and included large ulcers with surrounding friable tissue and no suitable omentum for patch repair. One was found to have an anastomotic leak at relaparotomy and the other died before undergoing a relaparotomy. The patient who had a repair done over a t-tube was found to have a duodenal ulcer surrounded by friable tissue and no viable omentum for a patch. The patient never required a relaparotomy and the t-tube was removed a few days later; the patient was discharged home in a stable condition. These numbers were too small to draw any significant conclusions on gastrectomy or t-tube repair for PPU.
The main limitation of this study was the reliance on the doctors attending to the patients to adequately enquire and capture the relevant history and risk factors pertaining to PPU at the time of admission. This was also pertinent at the time of surgery when the location of the ulcer was described, as many individuals still find it difficult to distinguish between pre-pyloric and duodenal ulcers. Furthermore, we could not evaluate those individuals who did not undergo relaparotomy with regard to whether or not their repair was intact or not. This could not be avoided as this was a retrospective study. Despite these limitations, we feel that the data presented provide valuable insights into the variable clinical courses of patients with PPU in our setting.
Conclusion
Based on our findings, clinicians should consider adding age greater than forty to the Boey score when risk stratifying patients with PPU on admission. Further research is required to investigate the impact of gender and ethnicity on mortality. A Boey score of > 1 was significantly associated with a leaking repair. Non-operative management remains a viable option in appropriately selected patients. Omental patch repair is a safe and effective surgical strategy for PPU, and is the most common management strategy in our setting.
Conflict of interest
The authors declare no conflict of interest.
Funding source
No funding was required.
Ethical approval
This study was approved by the University of KwaZulu-Natal Biomedical Research Ethics Committee (BREC) as part of completion of the MMed of Dr Jerome Nanack on 4 January 2022. BREC/00003678/2021.
ORCID
JJ Nanack https://orcid.org/0000-0003-1596-290X
L Ferndale https://orcid.org/0000-0003-1644-3124
REFERENCES
1. Lau JY, Sung J, Hill C, et al;. Systematic review of the epidemiology of complicated peptic ulcer disease: incidence, recurrence, risk factors and mortality. Digestion. 2011;84(2):102-13. https://doi.org/10.1159/000323958. [ Links ]
2. Xie X, Ren K, Zhou Z, Dang C, Zhang H. The global, regional and national burden of peptic ulcer disease from 1990 to 2019: a population-based study. BMC Gastroenterol. 2022;22(1):1-13. https://doi.org/10.1186/s12876-022-02130-2. [ Links ]
3. Lay PL, Huang HH, Chang WK, et al. Outcome of nonsurgical intervention in patients with perforated peptic ulcers. Am J Emerg Med. 2016;34(8):1556-60. https://doi.org/10.1016/j.ajem.2016.05.045. [ Links ]
4. Lanas A, Chan FKL. Peptic ulcer disease. Lancet. 2017;390(10094):613-24. https://doi.org/10.1016/S0140-6736(16)32404-7. [ Links ]
5. Bertleff MJ, Lange JF. Perforated peptic ulcer disease: a review of history and treatment. Dig Surg. 2010;27(3):161-9. https://doi.org/10.1159/000264653. [ Links ]
6. Ciftci F, Erözgen F. Patients with perforated peptic ulcers: risk factors for morbidity and mortality. Int Surg. 2019;103(11-12):578-84. https://doi.org/10.9738/INTSURG-D-15-00180.1. [ Links ]
7. Peiffer S, Pelton M, Keeney L, et al. Risk factors of perioperative mortality from complicated peptic ulcer disease in Africa: systematic review and meta-analysis. BMJ Open Gastroenterol. 2020;7(1):e000350. https://doi.org/10.1136/bmjgast-2019-000350. [ Links ]
8. Oribabor FO, Adebayo BO, Aladesanmi T, Akinola DO. Perforated duodenal ulcer; management in a resource poor, semi-urban Nigerian hospital. Niger J Surg. 2013;19(1):13-135. [ Links ]
9. Søreide K, Thorsen K, Harrison EM, et al. Perforated peptic ulcer. Lancet. 2015;386(10000):1288-98. https://doi.org/10.1016/S0140-6736(15)00276-7. [ Links ]
10. Thorsen K, Sereide JA, Sereide K. Scoring systems for outcome prediction in patients with perforated peptic ulcer. Scand J Trauma Resusc Emerg Med. 2013;21(1):1-10. https://doi.org/10.1186/1757-7241-21-25. [ Links ]
11. Maller MH, Engebjerg MC, Adamsen S, Bendix J, Thomsen RW. The peptic ulcer perforation (PULP) score. Acta Anaesthesiol Scand. 2012;56(5):655-62. https://doi.org/10.1111/j.1399-6576.2011.02609.x. [ Links ]
12. Menekse E, Kocer B, Topcu R, et al. A practical scoring system to predict mortality in patients with perforated peptic ulcer. World J Emerg Surg. 2015;10(1):1-6. https://doi.org/10.1186/s13017-015-0008-7. [ Links ]
13. Boey J, Choi SKY, Alagaratnam TT, Poon A. Risk stratification in perforated duodenal ulcers: a prospective validation of predictive factors. Ann Surg. 1987;205(1):22-26. https://doi.org/10.1097/00000658-198701000-00005. [ Links ]
14. Patel S, Kalra D, Kacheriwala S, Shah M, Duttaroy D. Validation of prognostic scoring systems for predicting 30-day mortality in perforated peptic ulcer disease. Turkish J Surg. 2019;35(4):252-8. https://doi.org/10.5578/turkjsurg.4211. [ Links ]
15. Caldwell RI, Gaede B, Aldous C. Description of an internal medicine outreach consultant appointment in Western KwaZulu-Natal, South Africa, 2007 to mid-2014. S Afr Med J. 2015;105(5):353-6. https://doi.org/10.7196/SAMJ.9173. [ Links ]
16. Chung KT, Shelat VG. Perforated peptic ulcer: an update. World J Gastrointest Surg. 2017;9(1):1. https://doi.org/10.4240/wjgs.v9.i1.1. [ Links ]
17. Tarasconi A, Coccolini F, Biffl WL, et al. Perforated and bleeding peptic ulcer: WSES guidelines. World J Emerg Surg. 2020;15(1):1-24. https://doi.org/10.1186/s13017-019-0283-9. [ Links ]
18. Seyoum N, Ethicha D, Assefa Z, Nega B. Risk factors that affect morbidity and mortality in patients with perforated peptic ulcer diseases in a teaching hospital. Ethiop J Health Sci. 2020;30(4):549-58. https://doi.org/10.4314/ejhs.v30i4.10. [ Links ]
19. Yalcin M, Oter S, Akmoglu A. Peptik ülser perforasyonunda erken ameliyat sonrasi morbidite ve mortalite belirleyicileri. Ulus Travma Acil Cerrahi Derg. 2022;28(11):1558-62. https://doi.org/10.14744/tjtes.2022.85686. [ Links ]
 Correspondence:
Correspondence:
email: jerome.nanack@gmail.com














