Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Surgery
versão On-line ISSN 2078-5151
versão impressa ISSN 0038-2361
S. Afr. j. surg. vol.61 no.4 Cape Town 2023
http://dx.doi.org/10.36303/sajs.4019
GENERAL SURGERY
Adverse events associated with the use of indwelling devices in surgical patients
AB WainI; S WallI; DL ClarkeI, II
IDepartment of Surgery, University of KwaZulu-Natal, South Africa
IIDepartment of Surgery, University of the Witwatersrand, South Africa
ABSTRACT
BACKGROUND: Indwelling devices (IDs) are ubiquitous in modern healthcare and may often be associated with morbidity. This paper investigates adverse events related to IDs in surgical patients, which are generally placed into patients either to administer therapy, manage outputs or for specific therapeutic benefit.
METHODS: A retrospective electronic database-based assessment of all adverse events relating to IDs was undertaken from December 2012 to August 2021. All events were categorised by device type, event type, and event severity.
RESULTS: A total of 11 130 morbidities were captured over the study period. Of those, 2 195 entries pertained to an ID with 2 402 reported adverse events affecting 1 592 patients. Two-thirds occurred in males and injuries occurred in patients age ranging from eight days to 93 years, with an average age of 36 years. The most frequently implicated devices were surgical drains (including intercostal chest drains), accounting for 491 (20.44%) of adverse events. Central venous catheters (CVCs) and intravenous cannulae were involved in 374 (15.57%) and 332 (13.83%) events, respectively. Unplanned removal (346, 13.91%), output not measured (319, 12.82%), injury (314, 12.62%), and blockage (279, 11.21%) were the most common error types. The majority of adverse events were considered minor, however 27 (1.1%) patients experienced organ dysfunction as a result of an ID-related adverse event, and seven (0.3%) died.
CONCLUSION: Morbidity related to IDs in surgical patients is a relatively frequent occurrence. Standardisation of ID insertion and care, staff education, and improvements in communication have been identified as the most important strategies by which we can limit error-associated morbidity in patients with IDs.
Keywords: adverse events, complications, central venous catheters (CVC), drain, peripherally inserted venous cannula (PIVC), error.
Introduction
The publication of To Err is Human at the turn of the millennium highlighted the fact that significant harm is incurred by patients secondary to human error and adverse events in health care.1 This contributed to the patient safety movement and encouraged several interventions designed to promote patient safety. Despite these efforts, the modern healthcare environment remains a relatively unsafe one when compared with other high-risk settings, such as the aviation industry and nuclear submarines.2 The proposed strategies to eliminate or reduce human error and adverse events in health care have included operative checklists, the development of early warning systems, mechanical marking of operative sites, and enforced protocols. Whilst these efforts have assisted in reducing unintentional harm in surgical care, they are not completely failsafe. Healthcare workers with varying skill and experience levels interact with a diverse patient cohort that demonstrate differing demographic and disease profiles, introducing multiple variables into an unpredictable and imperfect system. In light of this, it is important to capture as much data as possible about human error and adverse events within the healthcare system. Analysis of this aggregated data may allow for a deeper understanding of causation and contributing factors to help develop evidence-based strategies that reduce both the incidence of, and the impact of, error and adverse events.
Indwelling devices (IDs) are ubiquitous in modern health care. They include peripherally inserted venous catheters, centrally inserted venous catheters, urinary catheters, surgical drains, intercostal chest drains (ICDs), endotracheal tubes, tracheostomy tubes and nasogastric tubes. They are generally placed into patients either to administer fluids or antibiotics, facilitate controlled drainage or for monitoring purposes. Adverse events associated with these devices are well known and have been widely reported. Cooke et al. conducted a large cross-sectional survey in 2018 looking at consumer perspectives on the insertion of a peripherally inserted venous cannula (PIVC). They reported that while approximately 70% of inpatients require a PIVC, patient surveys regarding PIVC indicated that the insertion of even simple peripheral inserted venous (IV) line can result in significant distress.3 The same applies to almost all other IDs which are used commonly in modern health care. Our institution has maintained an electronic medical record system for a decade. This system captures data on all surgical admissions and has a dedicated platform to capture morbidity. This allows for the accrual of a significant data set pertaining to human error and adverse events in surgery. Following a review of almost ten years' data, this paper aims to classify adverse events that occur due to the use of IDs and document their severity. We hope to further our understanding of adverse events in surgical care, and to better comprehend the contribution of human error to these adverse events.
Methods
The Hybrid Electronic Medical Registry (HEMR) is an electronic database of all patients admitted to the Department of Surgery at Greys Hospital, Pietermaritzburg, South Africa. This system has been functional since December 2012. All data is covered by class approval from the Biomedical Research Ethics Committee (BREC) of the University of KwaZulu-Natal (BCA 221/13). All morbidity data between December 2012 and August 2021 were extracted for analysis. Captured morbidities were included for all ages, both genders, and all admitting surgical services (general surgery, trauma surgery, and paediatric surgery). Once adverse events related to the use of ID were identified, further analysis was undertaken. Each event was categorised by type of device involved, the type of adverse event, as well as according to the Clavien-Dindo classification system.
Results
A total of 11 130 morbidities were captured for the period December 2012 to August 2021. Of those, 2 195 entries (19.7% of all morbidities) pertained to ID use. These 2 195 entries encompassed 2 402 adverse events and affected 1 592 patients (in several instances there were multiple adverse events noted for a single patient on a single day). Of the 2 195 entries, 1 440 (65.6%) occurred in male patients, and 755 (35.4%) in females. Injuries occurred in patients of ages ranging from eight days to 93 years, with an average age of 36 years. Analysis by the admitting unit revealed that 1 234 (56.2%) were admitted under general surgery, 851 (38.7%) by trauma surgery, and 108 (4.9%) by paediatric surgery.
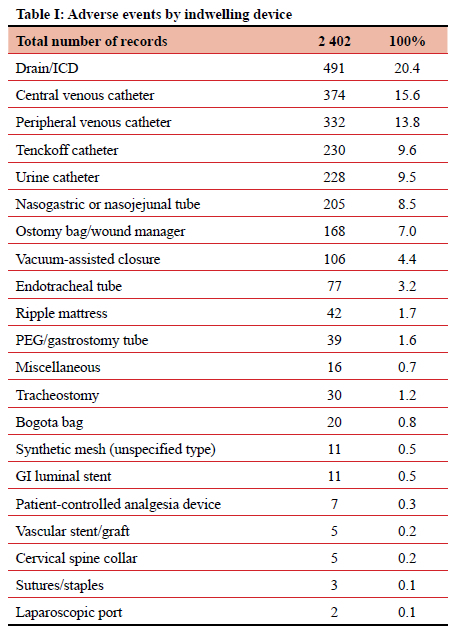
Indwelling device type
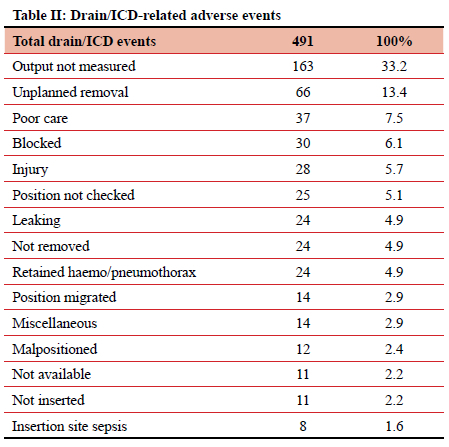
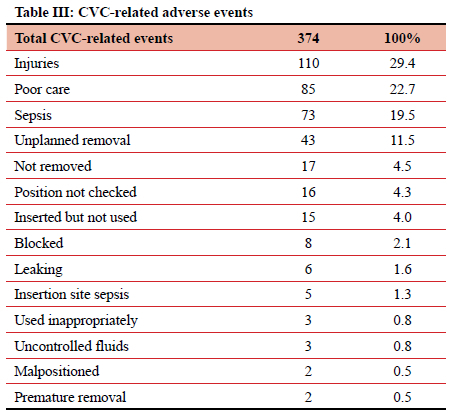
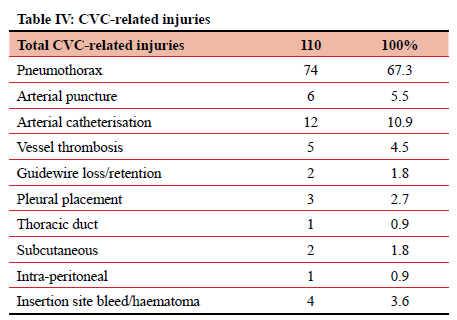
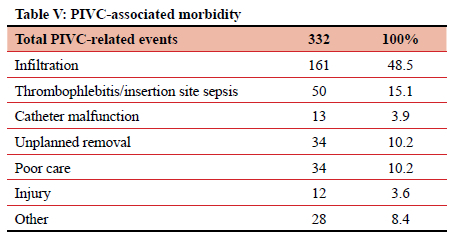
When divided by type, the most frequently implicated ID were surgical drains (including ICDs), accounting for 491 (20.44%) of 2 402 adverse events. Central venous catheters (CVCs) and PIVCs were involved in 374 (15.57%) and 332 (13.83%) of events, respectively (Table I). Of those 491 events relating to surgical drains, 163 (33.2%) were due to the output not being measured, and 66 (13.4%) due to unplanned removal (Table II). CVC-related adverse events totalled 394, contributing 15.6% of the total. In this study it was noted that 73 (19.5%) instances of CVC-related sepsis occurred, and 110 (29.4%) injuries resulted from the insertion or attempted insertion of a CVC. Of the 110 injuries resulting from CVC insertion, 67.3% were pneumothorax insertions (74 of 110), and 12 (10.9%) were an arterial catheterisation. There were 85 (22.7%) instances of poor CVC care (dressing soiled or loose, uncapped port, line not flushed, CVC not properly secured), 43 (11.5%) patients in whom the CVC was removed unintentionally, either by the patient or by staff, and 16 (4.3%) cases where the position of a new CVC was not confirmed radiologically before use. In 17 (4.5%) patients a CVC was left in situ for at least one additional day due to the line not being removed as planned (Tables III and IV). PIVCs accounted for 332 (13.8%) adverse events in this study. Of the 332 total events, 161 (48.5%) were due to infiltration into the soft tissues, 50 (15.1%) due to thrombophlebitis, and 34 (10.2%) due to unplanned removal (Table V).
Adverse events type
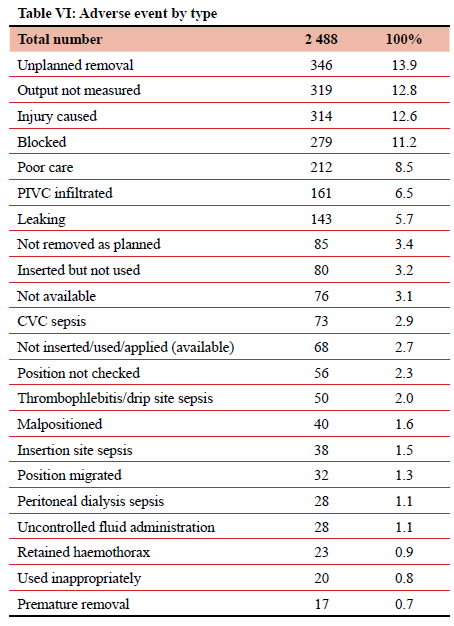
Analysis by morbidity type is reflected in Table VI. The most common adverse event types were unplanned removal (346, 13.91%), output not being measured (319, 12.82%), injury (314, 12.62%), and blockage (279, 11.21%). There were 346 (13.9%) instances of unplanned removal of an ID. Among this 'unplanned removal' category, the predominant adjuncts were nasogastric or nasojejunal tubes (NGT/NJT), surgical drains/ICDs, urinary catheters, CVCs, and endotracheal tubes (ETTs), comprising 91 (26.3%), 66 (19%), 49 (14.2%), 43 (12.4%) and 37 (10.7%) respectively. The remaining 60 (17.3%) were contributed to by PIVCs, gastrostomy tubes, vacuum-assisted closure (VAC) devices, and tracheostomy tubes. Injury as a result of ID use was documented to have occurred 314 times over the study period; 12.6% of the total ID-related adverse events. Of those 314, 110 (35%) were due to CVCs and 61 (19.4%) were due to transurethral catheters (TUCs). The majority of the TUC removals were by the patients themselves, either in a confused state, or accidentally. A total of 319 (12,8%) adverse events detailing 'output not measured' were documented in this study. Of those, the majority are due to outputs from drains/ICDs (163, 51.1%), stomas (66, 20.7%), and catheters (63, 19.7%).
Clavien-Dindo classification
According to the Clavien-Dindo classification, half of the adjunct-related morbidities were minor, or grade I (1 293, 53.8%) complications. However, 370 (15.4%) patients required corrective invasive intervention (grade IIIa), and a further 310 (12.9%) patients required intervention under general anaesthesia (grade IlIb) to manage the ID-related complication. A total of 27 (1.1%) patients experienced organ dysfunction as a result of an ID-related adverse event, with the respiratory and renal systems being most frequently affected. Seven (0.3%) patients demised secondary to the ID-related complication.
Discussion
The significant morbidity associated with the frequent use of IDs in surgical patients requiring inpatient care is highlighted in this retrospective review.
One in five adverse events in this study related to a surgical drain. Just under half of all surgical drain-related events necessitated a corrective intervention. There are two main errors associated with surgical drains, namely errors related to recording the output of a surgical drain and errors related to unplanned removal of a surgical drain. Accurate output reporting is important as clinical decision-making depends on accurate documentation. Repeated nursing education and training is needed to emphasise the importance of adequate documentation of surgical drain output. Unplanned removal of a surgical drain accounted for 13% of all surgical drain-related morbidity. Unplanned removal may be secondary to poor fixation, inadvertent removal by a patient, and communication errors between staff. Standardisation of fixation technique by protocol is important and communication between staff members should be improved. Improved written documentation to support verbal orders, and the use of information feedback systems, may contribute to reducing morbidity associated with inappropriate and inadvertent removal of surgical drains.
CVCs are associated with one in six adverse events. Insertion associated injury was the most common CVC-related adverse event, of which the most frequent were pneumothorax and arterial injury. This is in keeping with multiple publications documenting the spectrum of CVC-related injuries. A variety of methods have been employed to limit both the incidence and severity of these injuries. These include real-time ultrasonography, simulation-based training, and manometry.4-13 Poor care of the CVC was associated with 22.7% of adverse events. These included inadequate dressing of the CVC insertion site or uncapped and/or unflushed CVC ports. These are risk factors for CVC-associated blood stream infection (CLABSI). One-fifth of CVC-associated adverse events were due to CLABSI. Most centres attempt to reduce CLABSI by training on insertion and care techniques, and the use of care bundles for patients with CVCs.4-71013-15 These measures have lowered CLABSI rates.16,17
Peripheral intravenous cannulation is the most common invasive procedure in hospitalised patients and about 70% of inpatients will require a PIVC during their hospital stay.3 There is a high rate of PIVC-associated complications and this infers both significant morbidity and cost. PIVC morbidity is related to accidental removal, pain, infection, phlebitis, infiltration, and occlusion.3 In our study, 13.8% of all adjunct-related adverse events are associated with a PIVC. This rate almost certainly represents gross under-reporting. PIVC infiltration accounts for half of recorded PIVC-related adverse events. This is similar to the rate documented in other publications.3,18 PIVC-related infection accounts for 15% of all PIVC-related adverse events. Strategies to limit PIVC-related morbidity include care bundles, and routine site changes. The most practical approach is one of heightened site inspection to identify erythema or tenderness around the insertion site.3,18,19
Patients with an ID are at risk of several adverse events. Although not all of these adverse events are related to human error, several types of error have been identified. The main errors are associated with device insertion, fixation, output monitoring, and unplanned removals. There are four potential interventions, namely insertion-related training and standardisation, adherence to care bundles, staff education and improved communication between staff members. These interventions will need to be introduced as a multi-faceted strategy, if they hope to be effective in reducing IDassociated morbidity.
Conclusion
IDs are ubiquitous in modern health care and are associated with significant morbidity. Standardisation of ID insertion and care, staff education, and improved communication may help reduce this morbidity.
Conflict of interest
The authors declare no conflict of interest.
Funding source
No funding has been applied for or received for this work.
Ethical approval
Ethical approval has been granted by the Biomedical Research Ethics Committee of the University of KwaZulu-Natal, BREC/00004792/2022.
ORCID
AB Wain https://orcid.org/0000-0002-6693-0062
S Wall https://orcid.org/0000-0002-3935-9139
DL Clarke https://orcid.org/0000-0002-8467-1455
References
1. Donaldson MS, Corrigan JM, Kohn LT, editors. To err is human: Building a safer health system. Washington (DC): National Academies Press (US); 2000. [ Links ]
2. Gaba DM. Structural and organisational issues in patient safety: A comparison of health care to other high-hazard industries. Calif Manage Rev. 2000;43(1):83-102. https://doi.org/10.2307/41166067. [ Links ]
3. Cooke M, Ullman AJ, Ray-Barruel G, et al. Not "just" an intravenous line: Consumer perspectives on peripheral intravenous cannulation (PIVC). An international cross-sectional survey of 25 countries. PloS One. 2018;13(2):e0193436. https://doi.org/10.1371/journal.pone.0193436. [ Links ]
4. McGee DC, Gould MK. Preventing complications of central venous catheterisation. N Engl J Med. 2003;348(12):1123-33. https://doi.org/10.1056/NEJMra011883. [ Links ]
5. Safety Committee of Japanese Society of Anesthesiologists. Practical guide for safe central venous catheterisation and management 2017. J Anesth. 2020;34(2):167-86. https://doi.org/10.1007/s00540-019-02702-9. [ Links ]
6. Levy ER, Hutchins KA, Schears GJ, Rodriguez V, Huskins WC. How we approach central venous catheter safety: amultidisciplinary perspective. J Pediatric Infect Dis Soc. 2020;9(1):87-91. https://doi.org/10.1093/jpids/piz096. [ Links ]
7. Frampton GK, Harris P, Cooper K, et al. Educational interventions for preventing vascular catheter bloodstream infections in critical care: evidence map, systematic review and economic evaluation. Health Technol Assess. 2014;18(15):1-365. https://doi.org/10.3310/hta18150. [ Links ]
8. Fragou M, Gravvanis A, Dimitriou V, et al. Real-time ultrasound-guided subclavian vein cannulation versus the landmark method in critical care patients: a prospective randomised study. Crit Care Med. 2011;39(7):1607-12. https://doi.org/10.1097/CCM.0b013e318218a1ae. [ Links ]
9. Evans LV, Dodge KL. Simulation and patient safety: evaluative checklists for central venous catheter insertion. BMJ Qual Saf. 2010;19(Suppl 3):i42-46. https://doi.org/10.1136/qshc.2010.042168. [ Links ]
10. Smith RN, Nolan JP. Central venous catheters. BMJ. 2013;347. https://doi.org/10.1136/bmj.f6570. [ Links ]
11. Lamperti M, Bodenham AR, Pittiruti M, et al. International evidence-based recommendations on ultrasound-guided vascular access. Intensive Care Med. 2012;38(7):1105-17. https://doi.org/10.1007/s00134-012-2597-x. [ Links ]
12. Barsuk JH, McGaghie WC, Cohen ER, O'Leary KJ, Wayne DB. Simulation-based mastery learning reduces complications during central venous catheter insertion in a medical intensive care unit. Crit Care Med. 2009;37(10):2697-701. https://doi.org/10.1097/00003246-200910000-00003. [ Links ]
13. O'Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52(9):e162-93. https://doi.org/10.1093/cid/cir257. [ Links ]
14. Schulman J, Stricof R, Stevens TP, et al. Statewide NICU central-line-associated bloodstream infection rates decline after bundles and checklists. Pediatrics. 2011;127(3):436-44. https://doi.org/10.1542/peds.2010-2873. [ Links ]
15. Sun Y, Bao Z, Guo Y, Yuan X. Positive effect of care bundles on patients with central venous catheter insertions at a tertiary hospital in Beijing, China. J Int Med Res. 2020;48(7):0300060520942113. https://doi.org/10.1177/0300060520942113. [ Links ]
16. Karapanou A, Vieru A-M, Sampanis MA, et al. Failure of central venous catheter insertion and care bundles in a high central line-associated bloodstream infection rate, high bed occupancy hospital. Am J Infect Control. 2020;48(7):770-6. https://doi.org/10.1016/j.ajic.2019.11.018. [ Links ]
17. Odendaal J, Kong VY, Sartorius B, et al. Mechanical complications of central venous catheterisation in trauma patients. Ann R Coll Surg Engl. 2017;99(5):390-3. https://doi.org/10.1308/rcsann.2017.0022. [ Links ]
18. Braga LM, Parreira PM, Oliveira AdSS, et al. Phlebitis and infiltration: vascular trauma associated with the peripheral venous catheter. Rev Lat Am Enfermagem. 2018;26. https://doi.org/10.1590/1518-8345.2377.3002. [ Links ]
19. Catney MR, Hillis S, Wakefield B, et al. Relationship between peripheral intravenous catheter dwell time and the development of phlebitis and infiltration. J Infus Nurs. 2001;24(5):332-41. https://doi.org/10.1097/00129804-200109000-00008. [ Links ]
 Correspondence:
Correspondence:
email: howardwain@icloud.com














