Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Surgery
On-line version ISSN 2078-5151
Print version ISSN 0038-2361
S. Afr. j. surg. vol.61 n.3 Cape Town 2023
http://dx.doi.org/10.36303/sajs.3930
PAEDIATRIC SURGERY
The clinical utility of PET/CT scan and tissue biopsy in the management and follow-up of paediatric Hodgkin lymphoma in South Africa
K HeymanI; G HymanII; R KoliaI; K NicholaidesI; V GovenderI; J McMasterIII; D HarrisonIII
IUnit for Undergraduate Medical Education, Faculty of Health Sciences, University of the Witwatersrand, South Africa
IIDepartment of General Surgery, Faculty of Health Sciences, University of the Witwatersrand, South Africa
IIIDepartment of Paediatric Surgery, Faculty of Health Sciences, University of the Witwatersrand, South Africa
ABSTRACT
BACKGROUND: In low- to middle-income countries (LMICs) like South Africa, there is a need to understand the clinical practices surrounding diagnosis and surveillance of paediatric Hodgkin lymphoma (HL) to reduce the burden on health systems. Understanding the clinical utility of PET/CT scans may decrease repeated tissue biopsies during disease surveillance
METHODS: This is a retrospective cohort study of patients aged less than 18 years treated for HL at Chris Hani Baragwanath Academic Hospital from 1 January 2009 to 31 December 2018
RESULTS: Fifty-four patients were included in the study; male-to-female ratio was 5:1 with a mean age of 9 years. Seventy per cent of patients (n = 38) received a PET/CT and tissue biopsy during their initial diagnostic workup, whereas 20.4% (n = 11) of patients received a PET/CT and tissue biopsy during surveillance. Tissue biopsy and PET/CT showed slight agreement (k = 0.14) in diagnosing relapsed disease during surveillance. The false negative rate for tissue biopsy during surveillance was 42.9%. Surveillance PET/CT showed a positive predictive value (PPV) of 66.7% and negative predictive value (NPV) of 100% when compared to tissue biopsy
CONCLUSION: This study is the first cohort to explore the clinical utility of PET/CT scans and tissue biopsies in a low-resourced setting. Our findings showed slight agreement between the modalities in diagnosing relapsed disease during surveillance. A portion of this discordance can be attributed to false negative tissue biopsy results. While the sample is limited, our findings are consistent with the high NPV of PET/CT scans of > 95% as is reported in the literature
Keywords: clinical utility, PET/CT, Hodgkin lymphoma, South Africa, low- and middle-income countries
Introduction
Paediatric lymphomas are a major cause of morbidity and mortality in children. Lymphomas are the second most common paediatric cancer in Africa, with approximately 25% of paediatric lymphoma cases occurring in Africa.1,2 This incidence is proportional to the African contribution to the total population. However, a paucity of cancer registries and the underdiagnosis of malignancies in low- and middle-income countries (LMICs) indicates that the reported incidence may be underestimated.3 Additionally, a high burden of infectious diseases linked to the development of certain lymphomas, such as human immunodeficiency virus (HIV), may result in an increased prevalence.4 There is a lack of consensus regarding the burden of paediatric Hodgkin lymphoma (HL) in South Africa. However, a recent study reported that between 2000 and 2010, at least 294 patients were diagnosed with HL lymphoma across 10 paediatric oncology sites.5
South Africa's overburdened healthcare system leads to delays in presentation and diagnosis of HL. This results in more advanced stage III or IV and extra-nodal lymphomatous disease, and thus, an exacerbation in mortality.6-8 This places a great demand for investigations and treatment regimens on an under-resourced system. These factors highlight the need to determine the clinical utility of diagnostic modalities, such as PET/CT, in order to utilise available tools effectively. By utilising 18 fluorodeoxyglucose positron emission tomography/computed tomography (18FDG PET/CT) optimally, the number of invasive diagnostic procedures, such as excisional biopsies or the recommended less invasive core needle biopsies, performed by healthcare workers may decrease and health system sustainability may improve.9,10
There is a lack of consensus regarding the practices surrounding the diagnosis and surveillance of paediatric lymphomas, however, the National Cancer Institute (NCI) guidelines for HL recommend using imaging as a baseline investigation for suspected HL.11 18FDG PET/CT adds benefit in preventing over- or undertreatment when used in diagnosis and surveillance in HL.12 18FDG PET/CT has a negative predictive value (NPV) ranging between 90-95%, however a less impressive positive predictive value (PPV) of roughly 25% for HL. PET/CT is, however, more sensitive for determining the site of involvement of HL than conventional modalities, such as CT or magnetic resonance imaging (MRI).13,14 However, PET/CT shows some limitations in the paediatric population, as it may show tracer avidity for non-malignant conditions including excess brown fat in the neck region and post chemotherapy thymic rebound.15 Other reasons for false positive scans include inflammation, such as HIV and Epstein Barr virus (EBV) infections and in tumour necrosis.12,16,17 Therefore, following a positive scan, possible disease is then confirmed with a confirmatory tissue biopsy, the gold standard investigation.
Patient history, physical examination and blood tests are recommended for surveillance of relapsed disease. The routine use of imaging during this period is not yet recommended as the role of 18FDG PET/CT is still evolving and, thus, lacks a definitive place in diagnosis and surveillance. If relapsed disease is suspected, the initial diagnostic process will be repeated. In the South African setting, 18FDG PET/CT has been used in paediatric HL since 2005.18 However, it is not widely available in the public healthcare sector. Thus, its utility is not well established in the detection of paediatric HL.19 Within Gauteng, PET/CT is only available at Charlotte Maxeke Johannesburg Academic Hospital (CMJAH). Exploring the utility of PET/CT in the South African setting during surveillance may provide a basis for the expansion of access to the tool and inclusion of the tool in future management strategies.
Patients and methods
The study is a retrospective, secondary analysis. Data was collected from patients treated at Chris Hani Baragwanath Academic Hospital (CHBAH) in the departments of paediatric haematology and oncology and paediatric surgery from 1 January 2009 to 31 December 2018. The sample included patients aged 0-18 years old at the time who were histologically diagnosed with HL. Patients where the diagnosis of residual or relapsed disease was not made with either PET/CT or tissue biopsy were excluded. Variables included patient demographics, lymphoma subtypes, initial and surveillance diagnostic modalities and relapse outcomes. Follow-up and imaging modalities were decided by treating oncology clinicians; the research team could not retrospectively determine any objective tools used to determine which modalities were used.
Descriptive statistics were used to analyse patient demographics and histological subtype. Quantitative variables were characterised by measures of central tendency and dispersion. A Kaplan-Meier curve depicted patient survival analysis. A non-parametric Fisher's exact test measured the significance of the relationship between unpaired categorical variables. A p-value < 0.05 was deemed significant. Frequency tables assessed the accuracy of the initial and surveillance diagnostic investigations. A Cohen's kappa coefficient assessed the correlation between PET/CT and tissue biopsy results.
Results
Diagnosis and staging period
Sixty-one patients met our inclusion criteria, of which 88% (n = 54) of the patient records were included in our study. The remaining seven patient files were not located and excluded. The demographic data for the remaining cohort is depicted in Table I. A male preponderance is shown, and the median age of diagnosis was 8 years.
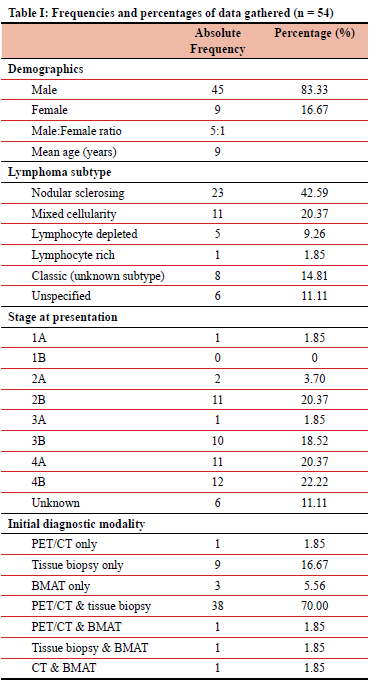
Of the 54 patients, 35.1% (n = 19) had HIV and 7.4% (n = 4) had comorbid EBV infection. Regarding the distribution of the histological subtypes of lymphoma, the majority of patients were diagnosed with nodular sclerosing lymphoma (Table I).
During initial staging, 62.9% (n = 34) of patients presented with advanced stages of HL, which was defined as stage 3 or greater. With regards to initial diagnostic modalities employed, most patients (70%, n = 38) had both a tissue biopsy and PET/CT scan with frequencies of diagnostic modality usage depicted in Table I.
In terms of relapsed disease, nodular sclerosing subtype was the most prevalent (44.44%, n = 4), however there was not a significant association between relapsed disease and initial subtype as per Fisher's exact test (p = 0.372). Patients who initially presented with advanced disease tended to present with relapsed disease during the surveillance period, however again this was not statistically significant as per Fisher's exact test (p = 0.297) (Table II).
In terms of complications post tissue biopsy, of the 88.9% (n = 48) of patients who underwent an initial tissue biopsy, 8.3% (n = 4) experienced complications due to infection. There were no statistically significant associations between initial biopsy complication and poor survival outcomes as per Fisher's exact test (p = 0.43).
Surveillance period
We defined the surveillance period as the two-year period following treatment completion. Sixty-one point one per cent (n = 33) underwent surveillance investigations, the pathway of which is depicted in Figure 1.
Tissue biopsy and PET/CT showed poor interrater reliability (k = 0.14), indicating slight agreement with a percentage of 48.2% (Table III). The false negative rate for tissue biopsy when compared to PET/CT was 42.9%. The PPV for surveillance PET/CT was 66.7% (95% CI 42.4%; 87.3%). The NPV was 100% (95% CI 81.6%; 100%).
Outcomes
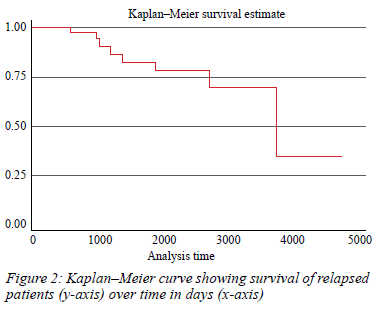
During the surveillance period, 16.67% (n = 9) of patients presented with relapsed disease. The median survival time for relapsed patients was approximately 8 years following diagnosis (Figure 2).
Discussion
This study provides an overview of the clinical utility of PET/CT scans compared to tissue biopsy in the diagnosis and surveillance of relapsed HL. Several differences were identified between the sample characteristics represented by this study and global trends. This study revealed a younger age range of 9 years ± 3.7 (o: ±3.7), when compared to high-income countries (HICs) where the average range of presentation is 15-19 years.18 This younger age of presentation is in keeping with data reported by other South African studies.620 The younger mean age at diagnosis in our cohort compared to HICs may be attributed to the increased risk factors, such as EBV and HIV, for HL in the population.4 Global trends show paediatric HL presents with a slight male predominance in HIC, however there is a large shift towards male predominance in LMICs with a ratio of 3:1.21 This study depicts a more significant male predominance with a male-to-female ratio of 5:1. A definitive reason for this finding cannot be explained by the aforementioned studies, nor by this study.
Nodular sclerosing subtype is the most common HL subtype reported in the United States, accounting for 80% of HL diagnoses in children. This is followed by mixed cellularity (20%), with the other subtypes being rare.22 However, the nodular sclerosing subtype is less prevalent among children in developing countries, only contributing to 55% of HL diagnoses.23 While the nodular sclerosing subtype was still the most common subtype in this study, it only contributed to 43% of patients (n = 23), thus showing lower prevalence as depicted in other developing countries. Fifteen per cent (n = 8) of our study's patients were only identified as having classic HL without a definitive subtype, with a further 11% (n = 6) as unspecified HL, thus the true incidence in our sample may vary.
While there is limited data on the stage at presentation in paediatric HL in developing countries, a Brazilian study conducted on adults with HL found that 65% of patients presented with advanced disease.24 A South African study by Geel et al. reported that 55% of their patients presented with advanced stage HL.5 Similarly, our study found that 62.9% (n = 34) presented with advanced disease. Potential contributing factors to the late stage at presentation include delayed diagnosis due to lack of access to healthcare and appropriate diagnostic tools.25 This further emphasises the importance of optimally utilising various diagnostic modalities.
Based on our data, patients initially diagnosed with nodular sclerosing lymphoma were most likely to experience a relapse, however this relationship was not significant (p = 0.372). Additionally, patients with an initial staging of 3B, 4A and 4B had a higher predisposition to relapse (p = 0.297). These findings are in keeping with a larger study that demonstrated relapse in patients with pretreatment staging of 2B, 3B, or 4 disease.26
There is consensus among the guidelines regarding the diagnostic value of PET/CT in the management of HL.13 PET/CT has a sensitivity and specificity of 80-95% and 90-95% respectively, and the NPV of PET/CT for excluding HL is 95-99%. However, PET/CT had limited capabilities in diagnosing relapsed disease, according to a study by Rhodes et al. which reported a PPV of 52%. Le Roux and colleagues report an even less impressive PPV ranging from 16-45%.27-29
Considering the limited sample size of our study, we were unable to reliably comment on the PPV and NPV of PET/CT scans when compared to tissue biopsy. A kappa statistic of 0.14, which corresponds to slight agreement, indicates that PET/CT and tissue biopsy only agreed 48.15% of the time when assessing relapsed or residual disease.
Two patients were treated for relapsed disease without repeat biopsies based on clinical examination, biochemistry and high FDG uptake on repeat PET/CT scans. There is no single agreed-upon threshold value of FDG uptake to definitively dictate the presence of relapsed or residual disease. A suggested cut-off point for a standardised uptake value in the primary tumour has ranged between 5-20 kBq/ ml.30 The above two patients in our study had uptakes of 9.63 kBq/ml and 7.29 kBq/ml respectively, both with recurrent disease close to their initial site of disease.
Of the patients who underwent repeat tissue biopsies during the surveillance period (n = 13), nine patients had a negative biopsy. However, three patients were still treated as relapses based on their positive PET/CT scans, clinical picture and bone marrow aspirate and tissue biopsy (BMAT). These false negative tissue biopsies may be due to obscuring reactive inflammatory cells, partial involvement of the sampled lymph node, sampling error and misinterpretation of results.31 This may necessitate a review of the usefulness of repeat tissue biopsy in our setting, where negative biopsies still result in patients being treated for relapse where their clinical condition or other investigations warranted further treatment. A possible explanation for the four false positive PET/CT results includes previous scar tissue and inflammation.
The role of combined diagnostic strategies to diagnose initial disease is clear. Seventy per cent (n = 38) of our sample set received both tissue biopsy and PET/CT, in keeping with the standard diagnostic procedure.31 In the event where a single diagnostic investigation was used for initial diagnosis in this cohort, a tissue biopsy only or a BMAT only were chosen more frequently than PET/CT alone. This is in keeping with the National Comprehensive Cancer Network (NCCN) guidelines which require histological confirmation for the initial diagnosis.32
A combination of PET/CT and BMAT was only used in one patient in our study. This combination is another possible diagnostic method that could be explored in future studies. This method would still be a viable option as it meets the NCCN guideline requirements for histological confirmation27 whilst sparing the child an invasive tissue biopsy.
Of the patients who underwent surveillance, PET/CT alone was used for 60.6% (n = 20) of patients, followed by a combination of tissue biopsy and PET/CT, which accounted for 33.33% (n = 11) of the sample. This shows that only 6% (n = 2) of patients undergoing surveillance did not receive a PET/CT scan, which speaks to good utilisation of PET/ CT as a surveillance tool. However, 42.7% of patients (n = 21) from the original cohort did not receive any surveillance investigations, as they were either lost to follow-up or demised.
It is clear from this study, as well as the literature, that the value of PET/CT for surveillance lies in its NPV. Clinically this means that asymptomatic patients with negative PET/ CT scans likely do not require further histological workup. Thus, the high level of utilisation and good NVP shown in this study highlight the clinical utility of this tool. However, due to its poor PPV and differences in the diagnostic outcomes between the two modalities (k = 0.14), PET/CT scans should not be used in isolation without a biopsy in patients with positive PET/CT results.
The lack of consensus regarding diagnostic modalities in this study reflect both the variability regarding the gold standard for diagnosis of relapsed HL, as well as the effect of resource limitations on HL care. While the sample size was too small to determine statistically significant relationships between diagnostic modalities and the diagnosis of relapsed disease, this study lays the foundation for future work in this field.
Study limitations
This study only considers a small sample population. Additionally, a considerable portion of our sample population (39%, n = 21) was lost to follow-up, thereby further limiting the sample size. The sample included in this study received treatment at CHBAH, a public health sector facility. This may have influenced the methods of diagnosis due to resource limitations. Additionally, access to PET/ CT in the public sector in itself limits the application of the findings due to the potential cost burden. CHBAH mainly services patients from Soweto as well as select patients without specialised care from other areas, thus creating a specific real area bias in our study. Finally, variables such as the cost of the diagnostic modality, time of hospital stay and time to diagnosis, which may influence clinical utility due to their impact on accessibility, were not considered.
Conclusion
This study is the first cohort study to explore the clinical utility of PET/CT scans and tissue biopsy in managing HL in a low-resourced setting. While the sample is small, the findings are consistent with the demographic and diagnostic data surrounding paediatric HL in developing countries. The findings from this study suggest that positive PET/CT scans should not be used in isolation in the surveillance of paediatric HL without biopsies, as there is only slight agreement between the two modalities. A portion of the discordance can be attributed to false negative tissue biopsy results of 42.9%. No patients had a negative PET/CT during surveillance with a positive tissue biopsy result. This highlights the importance of the NPV of PET/CT scans of > 95% as reported in the literature. However, the poor PPV of PET/CT scans limit their use in isolation. We recommend further research be undertaken to evaluate the clinical utility of PET/CT and the role of repeat tissue biopsy on a national level to improve the management and outcomes of paediatric HL in South Africa.
Acknowledgements
Dr Yvonne Perner, head of the department of anatomical pathology at the National Health Laboratory Service. Dr Sugeshnee Pather, senior lecturer at the department of anatomical pathology at the National Health Laboratory Service.
Fhatuwani Khaphathe, Noko Malatji, Phindulo Mavhina and Karl Allies for their contribution to data collection. The National Health Laboratory Service for the contribution of their data.
Conflict of interest
All authors declare no conflict of interest.
Funding source
No funding was required for this paper. Publication costs will be covered by the Department of Paediatric Surgery, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
Ethical approval
Ethical approval was obtained from the University of the Witwatersrand Human Research Ethics Committee (HREC) on 9 September 2021 (M217032).
ORCID
K Heyman https://orcid.org/0000-0003-1971-7477
G Hyman https://orcid.org/0000-0002-2541-0933
R Kolia https://orcid.org/0000-0002-5318-1277
K Nicholaides https://orcid.org/0000-0002-4985-8812
V Govender https://orcid.org/0000-0001-5356-3614
J McMaster https://orcid.org/0000-0001-5207-1642
D Harrison https://orcid.org/0000-0002-7093-1318
REFERENCES
1. Steliarova-Foucher E, Colombet M, Ries L, et al. International incidence of childhood cancer, 2001-10: a population-based registry study. Lancet Oncol. 2017;18(6):719-31. https://doi.org/10.1016/S1470-2045(17)30186-9. [ Links ]
2. The demographic profile of African countries [Internet]. Repository.uneca.org. 2022. Available from: https://repository.uneca.org/handle/10855/23177. Accessed 13 Jul 2022. [ Links ]
3. Johnston WT, Erdmann F, Newton R, et al. Childhood cancer: Estimating regional and global incidence. Cancer Epidemiol. 2021;71(Pt B):101662. https://doi.org/10.1016/j.canep.2019.101662. [ Links ]
4. Stefan C, Bray F, Ferlay J, Liu B, Maxwell Parkin D. Cancer of childhood in sub-Saharan Africa. Ecancermedicalscience. 2017;11:755. https://doi.org/10.3332/ecancer.2017.755. [ Links ]
5. Geel JA, Chirwa TC, Rowe B, et al. South African Children's Cancer Study Group. Treatment outcomes of children with Hodgkin lymphoma between 2000 and 2010: First report by the South African Children's Cancer Study Group. Paediatr Blood Cancer. 2017;64(10). https://doi.org/10.1002/pbc.26536. [ Links ]
6. Toobaie A, Emil S, Ozgediz D, Krishnaswami S, Poenaru D. Paediatric surgical capacity in Africa: Current status and future needs. J Paediatr Surg. 2017;52(5):843-8. https://doi.org/10.1016/j.jpedsurg.2017.01.033. [ Links ]
7. Padayachee RS, Perner Y, MacKinnon D, Rowe B, Pather S. A retrospective analysis of paediatric lymphomas at Chris Hani Baragwanath Academic Hospital in Soweto, South Africa. Ann Diagn Pathol. 2018;33:51-57. https://doi.org/10.1016/j.anndiagpath.2017.11.006. [ Links ]
8. Gini G, Cimminiello M, Galieni P, et al. Advanced stage Hodgkin lymphoma: patient management. Acta Biomed. 2020;91(S-5):5-12. [ Links ]
9. Groneck L, Quaas A, Hallek M, Zander T, Weihrauch MR. Ultrasound-guided core needle biopsies for workup of lymphadenopathy and lymphoma. Eur J Haematol. 2016;97(4):379-86. https://doi.org/10.1111/ejh.12742. [ Links ]
10. Krishnaswami S, Nwomeh BC, Ameh EA. The paediatric surgery workforce in low- and middle-income countries: problems and priorities. Semin Paediatr Surg. 2016;25(1):32-42. https://doi.org/10.1053/j.sempedsurg.2015.09.007. [ Links ]
11. PDQ Paediatric Treatment Editorial Board. Childhood Hodgkin Lymphoma Treatment (PDQ®) - health professional version. 2022 Apr 8. In: PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US); 2002-. [ Links ]
12. Flerlage JE, Kelly KM, Beishuizen A, et al. Staging evaluation and response criteria harmonisation (SEARCH) for childhood, adolescent and young adult Hodgkin lymphoma (CAYAHL): Methodology statement. Paediatr Blood Cancer. 2017;64(7). https://doi.org/10.1002/pbc.26421. [ Links ]
13. Naumann R, Vaic A, Beuthien-Baumann B, et al. Prognostic value of positron emission tomography in the evaluation of post-treatment residual mass in patients with Hodgkin's disease and non-Hodgkin's lymphoma. Br J Haematol. 2001;115(4):793-800. https://doi.org/10.1046/j.1365-2141.2001.03147.x. [ Links ]
14. Hudson MM, Krasin MJ, Kaste SC. PET imaging in paediatric Hodgkin's lymphoma. Paediatr Radiol. 2004;34(3):190-8. https://doi.org/10.1007/s00247-003-1114-3. [ Links ]
15. Cheson B. PET/CT in Lymphoma: Current overview and future directions. Semin Nucl Med. 2018;48(1):76-81. https://doi.org/10.1053/j.semnuclmed.2017.09.007. [ Links ]
16. Warwick J, Sathekge M. PET/CT scanning with a high HIV/ AIDS prevalence. Transfus Apher Sci. 2011;44(2):167-72. https://doi.org/10.1016/j.transci.2011.01.014. [ Links ]
17. Lustberg M, Aras O, Meisenberg B. FDG PET/CT findings in acute adult mononucleosis mimicking malignant lymphoma. Eur J Haematol. 2008;81(2):154-6. https://doi.org/10.1111/j.1600-0609.2008.01088.x. [ Links ]
18. Ellmann A. PET/CT in South Africa: a lost cause. SA J Radiol. 2008;12(3):58. https://doi.org/10.4102/sajr.v12i3.556. [ Links ]
19. Chambers G, Frood R, Patel C, Scarsbrook A. 18F-FDG PET-CT in paediatric oncology: established and emerging applications. Br J Radiol. 2019;92(1094):20180584. https://doi.org/10.1259/bjr.20180584. [ Links ]
20. Stefan DC. Hodgkin lymphoma in Africa: Present and future. Transfus Apher Sci. 2013;49(2):144-6. https://doi.org/10.1016/j.transci.2013.07.016. [ Links ]
21. Dinand V, Arya LS. Epidemiology of childhood Hodgkins disease: is it different in developing countries? Indian Paediatr. 2006;43(2):141-7. [ Links ]
22. Slack GW, Ferry JA, Hasserjian RP, et al. Lymphocyte depleted Hodgkin lymphoma: an evaluation with immunophenotyping and genetic analysis. Leuk Lymphoma. 2009;50(6):937-43. https://doi.org/10.1080/10428190902930488. [ Links ]
23. Bazzeh F, Rihani R, Howard S, Sultan I. Comparing adult and paediatric Hodgkin lymphoma in the Surveillance, Epidemiology and End Results Programme, 1988-2005: an analysis of 21 734 cases. Leuk Lymphoma. 2010;51(12):2198-207. https://doi.org/10.3109/10428194.2010.525724. [ Links ]
24. Biasoli I, Castro N, Delamain M, et al. Treatment outcomes for Hodgkin lymphoma: First report from the Brazilian prospective registry. Haematol Oncol. 2018;36(1):189-95. https://doi.org/10.1002/hon.2450. [ Links ]
25. Gaiolla RD. Hodgkin's lymphoma in developing countries: can we go further? Rev Bras Haematol Haemoter. 2017;39(4):299-300. https://doi.org/10.1016/j.bjhh.2017.08.004. [ Links ]
26. Smith RS, Chen Q, Hudson MM, et al. Prognostic factors for children with Hodgkin's disease treated with combined-modality therapy. J Clin Oncol. 2003;21(10):2026-33. https://doi.org/10.1200/JCO.2003.07.124. [ Links ]
27. Ferrari C, Niccoli Asabella A, Merenda N, et al. Paediatric Hodgkin lymphoma: predictive value of interim 18F-FDG PET/CT in therapy response assessment. Medicine (Baltimore). 2017;96(5):e5973. https://doi.org/10.1097/MD.0000000000005973. [ Links ]
28. Le Roux PY, Gastinne T, Le Gouill S, et al. Prognostic value of interim FDG PET/CT in Hodgkin's lymphoma patients treated with interim response-adapted strategy: comparison of International Harmonisation Project (IHP), Gallamini and London criteria. Eur J Nucl Med Mol Imaging. 2011;38(6):1064-71. https://doi.org/10.1007/s00259-011-1741-0. [ Links ]
29. Rhodes MM, Delbeke D, Whitlock JA, et al. Utility of FDG-PET/CT in follow-up of children treated for Hodgkin and non-Hodgkin lymphoma. J Paediatr Haematol Oncol. 2006;28(5):300-6. https://doi.org/10.1097/01.mph.0000212912.37512.b1. [ Links ]
30. Geus-Oei LF, Oyen WJ. Predictive and prognostic value of FDG-PET. Cancer Imaging. 2008;8(1):70-80. https://doi.org/10.1102/1470-7330.2008.0010. [ Links ]
31. Kaseb H, Babiker HM. Hodgkin lymphoma. [Updated 2022 Mar 19]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK499969/. [ Links ]
32. Hoppe RT, Advani RH, Ai WZ, et al. NCCN guidelines insights: Hodgkin lymphoma, version 1.2018. J Natl Compr Canc Netw. 2018;16(3):245-54. https://doi.org/10.6004/jnccn.2018.0013. [ Links ]
 Correspondence:
Correspondence:
K Heyman
Email: kellheyman@gmail.com














