Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Surgery
versión On-line ISSN 2078-5151
versión impresa ISSN 0038-2361
S. Afr. j. surg. vol.61 no.3 Cape Town 2023
http://dx.doi.org/10.36303/sajs.3903
HEPATOPANCREATICOBILIARY SURGERY
Geographic distribution of pancreaticobiliary malignancy in central South Africa presenting to the Universitas Academic Hospital Complex
RJ MthunziI; CB NoelII
IDepartment of Surgery, University of the Free State, South Africa
IIHepatopancreaticobiliary Clinical Unit, Department of Surgery, Universitas Academic Hospital, University of the Free State, South Africa
ABSTRACT
BACKGROUND: There is limited data on the epidemiology, determination of risk factors and geographical variation of pancreatic cancer in South Africa. The aim of this study is to describe these parameters within central South Africa and compare to national and international reports
METHODS: A retrospective review of all patients with newly diagnosed pancreatic cancer on clinical and radiological grounds admitted to Universitas Academic Hospital from 1st January 2015 to 31st December 2019 was performed. Patients were grouped into geographical regions based on their district municipality to identify clusters of pancreatic cancer. Demographic information and details of family history, diabetes and smoking status, and chronic pancreatitis were recorded and analysed in conjuction with the geographical and census data to provided estimates of disease incidence
RESULTS: The mean age of the the 382 patients with pancreatic cancer in the study period was 62.8 years ± 11.06. Two hundred and twelve (55.5%) were females. The Frances Baard district in the Northern Cape had the highest estimated rate of 3.5/100 000 and the Thabo Mofutsanyana district the lowest at 1.0/100 000. Of the cohort 132 (34.5%) were active smokers, 71 (18.6%) had diabetes mellitus, four (1%) had a history of chronic pancreatitis and two (0.5%) had a family history of pancreatic cancer
CONCLUSION: The incidence of pancreatic cancer in central South Africa is higher than that reported nationally with a female gender bias, marked regional variation and lack of a family history. These observations merit further evalualtion in the South African context
Keywords: pancreaticobiliary malignancy, geographic distribution
Introduction
The burden and prognosis of pancreatic cancer remains a concern. Currently, pancreatic cancer is the seventh most common cause of global cancer-related deaths, and is predicted to be the second most common cause by the year 2030. In addition, pancreatic cancer continues to have the worst 5-year mortality rate.1,2
In 2018, 458 918 new cases of pancreatic cancer were identified worldwide. Pancreatic cancer accounted for 432 242 deaths, with higher rates in developed countries globally. Hungary, Uruguay and Moldova had the highest rate of pancreatic cancer with rates of 10.8/100 000; 10.7/100 000 and 10.5/100 000 respectively. The lowest rates were observed in Africa, more specifically Guinea (0.35/100 000).1,2
According to the South African National Cancer Registry (NCR), pancreatic cancer is the 24th most common malignancy with a reported rate of 0.8/100 0000.3 This may not be a true representation as pancreatic cancer is under diagnosed and under reported in developing countries largely due to limited access to resources needed to make the diagnosis.
The aetiology of pancreatic cancer is not completely understood. Risk factors associated with pancreatic cancer include a strong family history of pancreatic cancer, chronic pancreatitis, diabetes mellitus, advancing age, smoking, westernised diet, male gender and genetics.4
There is increasing emerging global evidence that pancreatic cancer has an underlying genetic link and new techniques for screening of specific target groups are being evaluated.5 Genetic predisposition syndromes associated with an increased risk of developing pancreatic cancer include hereditary pancreatitis PRSS1 mutation, Peutz-Jeghers syndrome STK11 mutation, familial atypical mole and melanoma syndrome CDKN2A mutation, Lynch syndromes MMR genes (particularly MLH1, MSH2 and MSH6) and ataxia telangiectasia ATM mutation.5
Apart from tumour biology, numerous other factors play an integral role with regards to the poor prognosis associated with pancreatic cancer. Internationally, including developed countries, less than 20% of patients have resectable pancreatic cancer at presentation.2 The diagnostic challenge remains the inability to identify patients with pancreatic cancer in the early stages of the disease. With increasing evidence around a genetic predisposition, identifying geographical areas with a high incidence of pancreatic cancer will aid further research into identifying additional risk factors.
The most recent recommendations by the International Cancer of the Pancreas Screening (CAPS) Consortium advocate for the screening of pancreatic cancer in high risk groups from age 50 years or 10 years younger than the youngest relative with pancreatic cancer. However, the criteria for eligibility of screening were based on studying of existing genetic mutations and family history and no other risk factors.6
Baum et al. evaluated the regional variation of pancreatic cancer incidence in the Nile Delta region of Egypt. The study found that incidence rates for pancreatic cancer differed by districts. Incidence rates were significantly higher in more urban areas as compared to rural districts. Notably they hypothesised that the disparities could not be due to differences in access to health services in the two areas as 95% of Egypt's population lives within a five-kilometre distance to a primary health care facility. In addition, both pancreatic cancer treatment centres are less than fifty kilometres from these primary health care facilities.7
The Shandong province of China has one of the highest incidence rates of pancreatic cancer morbidity and mortality in China.8 In a review of the geographical distribution and clusters of pancreatic cancer mortality in the province, Jiang et al. demonstrated that higher mortality rates were mainly concentrated in the more developed eastern and northern regions of Shandong, while lower rates were found in the less developed western and central regions. They postulated that in addition to other common well-known risk factors, the higher mortality rate observed in the eastern region was possibly due to population ageing, which was more prevalent in the developed region.
The authors further revealed four different clusters of pancreatic cancer mortality, with the most likely cluster covering the north-eastern, northern and central regions. They concluded that identification of risk clusters of mortality can provide a basis for continued studying of geographical and environmental risk factors of the disease. In addition to devising prevention and control measures for pancreatic cancer in specific regions, the results of the study could contribute to the development of effective, targeted strategies to control pancreatic cancer in different areas.8
Of note, which is acknowledged in both studies, urbanisation is a process that is accompanied by socioeconomic development. The more developed the region, the more prevalent risk factors, such as increased smoking rates, adoption of westernised diets, lack of exercise and increased diabetes rates exist.7,8 The higher prevalence of these risk factors for pancreatic cancer seen in urbanised regions may account in part to the possible association found between urbanisation and pancreatic cancer.9,10
In a review of the epidemiology of pancreatic cancer in India, Rajshree et al. sought to elucidate the incidence, mortality and risk factors for pancreatic cancer in the Indian population. The study highlighted risk factors for pancreatic cancer and suggested that although screening of large populations may not be feasible, identification of high-risk groups is in order to target earlier detection and planning of prevention strategies.11
da Costa et al. examined trends in the incidence of pancreatic adenocarcinoma in all 50 states of the United States of America (USA). Incidence rates were higher in individuals older than 75 years, and in non-Hispanic black ethnic groups. Both groups also had higher mortality rates and metastatic disease at diagnosis. They observed similar rates among males and females despite prior studies in the USA reporting a male to female ratio incidence of 2:1.
Trends in incidence rates were varying amongst states, with some indicating a possible early decrease in incidence. In the states where a marked increase was shown, the simultaneous increase in the prevalence in diabetes mellitus, higher smoking rates and consumption of food with high sugar content were also noted.12 The above data reaffirms the notion that earlier identification of the disease is needed, and that age, smoking, diabetes mellitus and socioeconomic status should be taken into account in identifying a high-risk group. The concept that the above variables or risk factors for pancreatic cancer may be clustered to certain geographical areas is an attractive idea, in that it may then be of value in targeting a population for screening purposes.
To date there is no national screening programme for pancreatic cancer available in South Africa nor has any high-risk group been identified. No geographical mapping of the incidence of pancreatic cancer in a South African population group has been reported. This study aimed to identify the incidence of pancreatic cancer by geographical origin in central South Africa.
Methods
A retrospective review of a prospectively kept database of all patients admitted to Universitas Academic Hospital (UAH) hepatopancreaticobiliary (HPB) clinical unit from 01/01/2015-31/12/2019 was performed. All newly diagnosed patients with pancreatic cancer on clinical and radiological grounds were included. Those patients admitted with a known diagnosis of pancreatic cancer or those newly diagnosed but referred from outside of central South Africa were excluded.
Central South Africa includes the Free State province, the Northern Cape, western KwaZulu-Natal, and the northern section of the Eastern Cape.6 The UAH catchment area includes not only the above provinces, but also Lesotho. For the purpose of this study central South Africa was defined as Free State and the Northern Cape provinces.
The number of patients with pancreatic cancer were grouped into district municipalities which represented the geographical area and was used as the numerator over the five year data collection period. Data from statistics SA census of 2016 was used to obtain population size in each district municipality in order to obtain the denominator.13 Demographic data included in the study were age, gender and geographical address. Risk factors for pancreatic cancer included diabetes mellitus, smoking, family history of pancreatic cancer and chronic pancreatitis.
Approval for the collection of data was obtained from the Free State Department of Health. Ethical clearance for the study was granted by the Health Sciences Research Ethics Committee of the University of the Free State. The statistical analysis of the data collected was performed by the University of the Free State Department of Biostatistics using frequencies and percentages. The ^-values were calculated comparing percentages of females versus males using the chi-squared test.
Results
A total of 382 patients with pancreatic cancer were included; 212 (55.5%) patients were females and 170 (44.5%) were males. The mean age of all of the patients with pancreatic cancer was 62.8 years (standard deviation of ± 11.06). 240 (62.8%) patients with pancreatic cancer originated from the Free State and 142 (37.2%) patients were from the Northern Cape province.
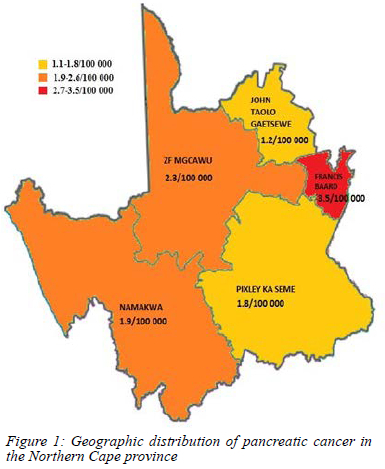
The highest rate of pancreatic cancer was reported in the Frances Baard district in the Northern Cape, with a rate of 3.5/100 000 population.14 The rates of pancreatic cancer in all the five districts are depicted in Figure 1.15
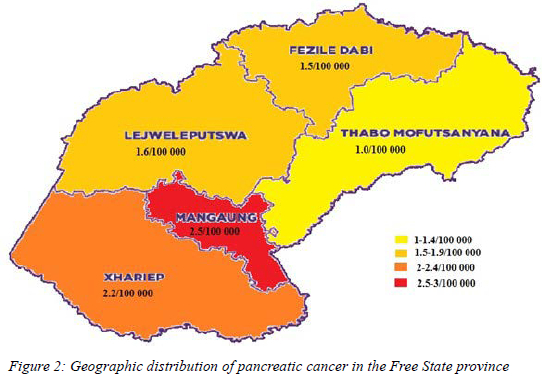
The second highest rate of pancreatic cancer of 2.5/100 000 was found in the Mangaung district within the Free State province. The comparative rates of the other four districts in the Free State province are depicted in Figure 2.16
44.5% of pancreatic cancer patients originated from the Northern Cape province. Of those 18.3% (n = 26) had diabetes mellitus with no significant difference between genders (12% of females versus 6.3% of males, p = 0.268), 38% (n = 54) were active smokers with a significant difference between genders (16.2% of females versus 21.8% of males, p = 0.0143) and none had a previous diagnosis of chronic pancreatitis. Two patients were recorded to have a family history of pancreatic cancer in the Northern Cape population with no significant difference between genders (0% of females versus 1.4% males, p = 0.1107).
62.8% of pancreatic cancer cases were from the Free State province, 18% (n = 45) of the patients had coexisting diabetes mellitus with no significant difference between genders (12.5% of females versus 6.25% of males, p = 0.0921), 32.5% (n = 78) were active smokers with a significant difference between genders (10.8% of females versus 21.7% of males, p < 0.0001), and 1.6% (n = 4) had a preexisting diagnosis of chronic pancreatitis with no significant difference between genders (0.8% of females versus 0.8% of males, p = 0.826). None of the patients diagnosed with pancreatic cancer originating from the Free State reported a family history of pancreatic cancer.
Discussion
This study is the first in South Africa to investigate the geographical distribution of pancreatic cancer. There are currently no high risk groups identified and no recommended screening programme in South Africa. Like risk factors, geographic distribution of incidence adds valuable information to the epidemiology of the disease. The NCR of South Africa reports pancreatic cancer as the 24th most common malignancy with a reported rate of 0.8/100 0000. All of the districts within central South Africa were found to have rates of pancreatic cancer that are far higher than the national reported incidence of pancreatic cancer. Global trends show that the diagnosis of pancreatic cancer is higher in geographical areas with older age population groups. The mean age of diagnosis of pancreatic cancer in the central South African population is 62.8 years, however the geographical areas in which the rate of pancreatic cancer was the highest had a population with a mean age of 25 years.1,8,12,13
This study demonstrated a higher incidence of pancreatic cancer in females than males which is contradictory to international studies that report a male to female ratio of 2:1.1,12 Our study found that 18% of the patients diagnosed with pancreatic cancer had coexisting diabetes mellitus. This is higher than 9.7% which is what is generally reported internationally.1,17
Of note is the fact that the highest incidence rate of pancreatic cancer (3.5/100 000) was found in the Frances Baard district within the Northern Cape. This number is substantially higher than reported in other African studies and 4.4 times higher than the incidence of 0.8/100 000 reported by the NCR of South Africa.1,3 The highest percentage of patients diagnosed with pancreatic cancer who smoked was found in the Francis Baard district (47%), with 17% of patients having coexisting diabetes.
Only two patients were recorded to have a family history of pancreatic cancer and both of them were found in the Northern Cape population. The lower than expected reported family history may be confounded by a number of reasons. There may be discrepancies in the records from consultation with patients who are poor historians or patients that are unsure of the existence of any family member who had previously been diagnosed with or died of pancreatic cancer.
Within the Free State the majority of patients were from the more urbanised Mangaung district.13,18,19 With urbanisation comes the adoption of a more westernised lifestyle, more smoking, less exercise and a high incidence of diabetes mellitus, all of which are known risk factors for the development of pancreatic cancer.7,8,11 This study confirmed this notion in a South African population group with Mangaung. 16.1% of patients diagnosed with pancreatic cancer in the Mangaung district had coexisting diabetes mellitus and 29.2% were active smokers.
Despite all pancreatic cancer diagnosed in central South Africa in the public sector being referred to UAH HPB unit, this study is limited in that only patients who were clinically well enough to undergo transport would have ultimately been included. Patients with very advanced disease would not therefore have been accounted for. In addition, the diagnosis of pancreatic cancer based on clinical and radiological features alone, as in this study, could have resulted in an overestimation of incidence. Due to discrepancies that can possibly occur in collection of census data including death registries and population counts, incorrect values in population statistics can prevent accurate measurements of the true incidence of disease in certain geographic areas.
Conclusion
The incidence of pancreatic cancer is higher than that reported in the South African population. This study shows that geographic variations of pancreatic cancer can be highlighted. We also highlighted the importance of knowing the risk factors that exist and that may contribute to high risk groups within regions. The large number of patients without a reported risk factor necessitates the need for further research into identifying other population specific risk factors.
Through these findings, the implementation of policies and strategies on screening for pancreatic cancer in regions with high risk individuals could be considered. Further research in identified high risk geographical areas will further contribute to the knowledge regarding the epidemiology and possible screening strategies in a South African contextualised population. Modelling of the risk factors in the population of central South Africa and their association with pancreatic cancer should be explored in order to assess the feasibility of such screening programmes. Lastly, this study identifies the need for further research into identifying the obstacles around accurate identification and possibly under-reporting of genetic and familial contributors to cancer predisposition diseases.
Acknowledgement
Prof. Gina Joubert of the Department of Biostatistics for her guidance in the planning, statistical analysis and write-up of the study. Dr O Aluko from the Department of Biostatistics for assistance in the statistical analysis of the data.
Conflict of interest
The authors declare no conflict of interest.
Funding source
No external source of funding was received during the conduct of the study.
Ethical approval
The study was approved by the Health Sciences Research Ethics Committee of the University of the Free State. Ethics number UFS-HSD2020/1021/2710.
ORCID
RJ Mthunzi https://orcid.org/0000-0003-2609-8977
CB Noel https://orcid.org/0000-0003-2344-415X
REFERENCES
1. Rawla P, Sunkara T, Gaduputi V. Epidemiology of pancreatic cancer: global trends, etiology and risk factors. World J Oncol. 2019;10(1):10-27. https://doi.org/10.14740/wjon1166. [ Links ]
2. Castillo CF, Jimenez, RE. Epidemiology and nonfamilial risk factors for exocrine pancreatic cancer. UpToDate. 2019;2480(60). [ Links ]
3. National Institute for Communicable Diseases. National Cancer Registry. RSA; 2014. [ Links ]
4. World Cancer Research Fund, American Institute for Cancer Research. Pancreatic cancer Statistics (USA); 2019(1). [ Links ]
5. Canto MI. Familial risk factors for pancreatic cancer and screening of high-risk patients. UpToDate. 2019;83943(21). [ Links ]
6. Goggins M, Overbeek KA, Brand R, et al. Management of patients with increased risk for familial pancreatic cancer: updated recommendations from the International Cancer of the Pancreas Screening (CAPS) consortium. BMJ. 2019;0:1-11. https://doi.org/10.1136/gutjnl-2019-319352. [ Links ]
7. Baum C, Soliman AS, Brown HE, et al. Regional variation of pancreatic cancer incidence in the Nile Delta region of Egypt over a twelve-year period. J Cancer Epidemiol. 2020;6031708:1-9. https://doi.org/10.1155/2020/6031708. [ Links ]
8. Jiang F, Chu J, Chen X, et al. Spatial distribution and clusters of pancreatic cancer mortality in Shandong province, China. Scientific Reports. Nature Research. 2019;9:1-7. https://doi.org/10.1038/s41598-019-49357-w. [ Links ]
9. World Health Organization. Nutritional Landscape Information System. Nutrition and nutrition-related health and development data 2020. Available from: https://www.who.int/data/nutrition/nlis/info/human-development-index. [ Links ]
10. Wong M, Jiang J, Liang M, et al. Global temporal patterns of pancreatic cancer and association with socioeconomic development. Sci Rep. 2017;7(1): 3165. https://doi.org/10.1038/s41598-017-02997-2. [ Links ]
11. Rajshree HG, Ganesh B. An epidemiological review of pancreatic cancer with special reference to India. Indian J Med Sci. 2021;73(1): 99-109. https://doi.org/10.25259/IJMS_92_2020. [ Links ]
12. Da Costa W, Oluyomi AO, Thrift AP. Trends in the incidence of pancreatic adenocarcinoma in all 50 United States examined through an age-period-cohort analysis. Oxford University Press. JNCI Cancer Spectrum. 2020;4 (4):1-7. https://doi.org/10.1093/jncics/pkaa033. [ Links ]
13. Department of statistics South Africa. Stats SA. Statistics by place. RSA; 2016. https://www.statssa.gov.za/?page_id=964. Accessed 08 September 2021. [ Links ]
14. Municipalities of South Africa. District municipalities. 2021. Available from: https://municipalities.co.za. Accessed 01 April 2022. [ Links ]
15. Travel in South Africa. Northern Cape. 2019. Available from: http://travelinsa.wordpress.com. Accessed 01 April 2022. [ Links ]
16. Travel in South Africa. Free State. 2019. Available from: http://travelinsa.wordpress.com. Accessed 01 April 2022. [ Links ]
17. Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res Clin Pract. 2019;157:107843. https://doi.org/10.1016/j.diabres.2019.107843. [ Links ]
18. Puuka J, Dubarle P, McKiernan H, et al. Higher education in regional and city development. The Free State, South Africa. OECD Publishing; 2012. Available from: https://www.oecd.org/education/imhe/50008631.pdf. Accessed 11 November 2022. [ Links ]
19. Department of Statistics South Africa. Investigation into appropriate definitions of urban and rural areas for South Africa; 2003. Available from: https://www.statssa.gov.za/?page_id=5134. Accessed 11 November 2022. [ Links ]
 Correspondence:
Correspondence:
RJ Mthunzi
Email: rmthunzi11@gmail.com














