Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Surgery
On-line version ISSN 2078-5151
Print version ISSN 0038-2361
S. Afr. j. surg. vol.61 n.2 Cape Town 2023
http://dx.doi.org/10.36303/SAJS.3899
CASE REPORT
Fungal abscess of the parotid gland - the value of microbiological assessment
AK Ogonowski Bizos; M White; J Fagan; V Pretorius
Otorhinolaryngology Division, Faculty of Health Sciences, University of Cape Town, South Africa
SUMMARY
Fungal parotitis is rare and the sequela parotid abscess exceedingly so. We report our experience with Candida glabrata and Candida albicans parotid gland abscesses in critically ill HIV-positive patients and highlight the value of microbiological assessment to tailor management.
Keywords: fungal abscess, parotid gland, microbiological assessment
Case 1
A 58-year-old male with a 6-month history of pain in the right temporomandibular joint presented in decompensated left ventricular failure. He had a fluctuant mass in the pre-auricular area associated with painful mastication. He had ischaemic heart disease, hypertension, cardiac failure and chronic kidney disease (CKD). He was HIV positive (CD4 count of 323 cell/Ul, viral load [VL] was lower than detectable [LDL]). He was admitted for medical management for the cardiac failure and was started on oral clindamycin, empirically awaiting microscopy, sensitivity and culture (MC&S) of the pre-auricular abscess aspirate.
The aspirate cultured a light growth of Candida glabrata sensitive to amphotericin B. Fine needle aspiration cytology (FNAC) of the mass documented the presence of acid-fluorescent bacilli along with degenerate inflammatory cells in a necrotic background. Based on the FNAC findings, he was started on empiric tuberculosis treatment but with no confirmed diagnosis of mycobacterium tuberculosis on culture and continued on tuberculosis treatment throughout his admission and on discharge. On discussion with microbiology, antifungal treatment was not initiated as it was thought to be a contaminant. He remained apyrexial on clindamycin and was continued on this antibiotic prior to referral to the ear, nose and throat (ENT) department. At that time, a tender, well-localised cystic swelling over the right parotid gland was present. There was extensive candidiasis in the oral cavity. The facial nerve was intact and there was no trismus or odontogenic sepsis. Oral fluconazole and oral nystatin were commenced to treat the extensive oral candidiasis while awaiting culture results of a second aspirate. This aspirate again yielded a moderate growth of C. glabrata, which was sensitive to amphotericin B. The infectious diseases department recommended treatment with oral fluconazole 200 milligrams (mg) daily to treat his oral candidiasis. A clinical review and repeat aspiration on day 13 yielded 2 ml of pus; thereafter there was complete resolution of the abscess. On day 25 of admission, the patient contracted COVID-19. He was transferred to a stepdown facility where he died.
Case 2
A 46-year-old female presented with multiple comorbidities including hypertension, poorly controlled type 2 diabetes mellitus (DM), HIV on antiretroviral therapy (CD4 count of 84 cell/Ul, VL of 303 copies/ml), and tuberculous meningitis on treatment.
She presented to the emergency unit acutely unwell, in a hyperosmolar hyperglycaemic state, precipitated by sepsis (pH 7,1, base excess -20, WCC 34). She had a week's history of a painful swelling at the left angle of the mandible.
She had clinical features of acute parotitis with an associated abscess, with intact facial nerve and no trismus.
She was also noted to have oral candidiasis and odontogenic sepsis. Contrasted CT scan revealed a large multi-loculated, rim-enhancing, intraparenchymal collection of the parotid, with associated sub-centimetre, reactive cervical lymphadenopathy, and no evidence of sialolithiasis (Figure 1).
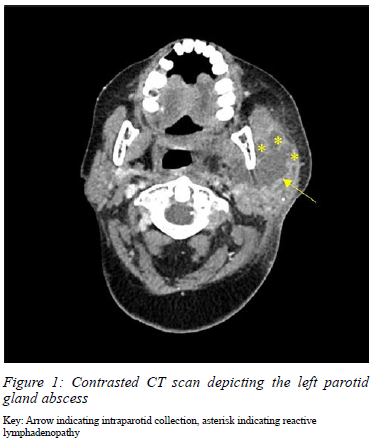
A bacterial suppurative parotid abscess was suspected, therefore amoxicillin/clavulanic acid therapy was initiated. She had an urgent incision and drainage (I&D) under general anaesthesia; 30 ml pus was drained from a collection involving both deep and superficial lobes of the parotid gland. Tissue specimens and pus were sent for MC&S, and both cultured Candida albicans sensitive to fluconazole. Histology from the abscess wall and parotid gland revealed moderate acute-on-chronic inflammation with no evidence of malignancy or granulomatous inflammation. Following consultation with the infectious diseases department on day 6 of admission, fluconazole was initiated and was continued for 1 month (400 milligrams daily po). She responded well to antifungal therapy and was discharged on day 14 with a small residual, granulating sinus at the site of previous I&D. At 2 months follow-up, she had complete resolution of the sinus and her surgical wound had healed with minimal scarring.
Discussion
The most common suppurative infections of the parotid gland parenchyma in adults are of bacterial origin.1-6 Fungal parotitis is extremely rare, with only 11 cases reported in the literature.3,5,8,9 Of these, six cases of fungal parotitis were caused by C. glabrata and four cases were caused by C. albicans.3,5,1-9
All C. glabrata parotitis cases resulted in subsequent parotid gland abscesses.6 Of the aforementioned cases, the ages ranged from 32 to 82 years. Underlying comorbidities included DM, HPT and CKD. Five of the six patients with C. glabrata parotitis were treated with fluconazole. All except one of the other patients with parotitis complicated with a parotid gland abscess due to C. glabrata fully recovered with a management approach of I&D, and fluconazole.3,5,8,9
Of the four previous cases presenting with suppurative parotitis due to C. albicans, three developed a parotid gland abscess. All were treated with fluconazole in addition to I&D with a resultant successful outcome; a negligible mortality rate has been reported. DM was the common underlying condition for all reported cases of C. albicans parotitis.4 Of note, only two cases are documented to have HIV co-infection as a comorbidity.
There are currently no formal guidelines for medical management of fungal abscesses specifically relating to the head and neck region.
A review of the literature of case reports of candida parotitis show a variation in the dosage and duration of antifungal agents. There are four cases of previous parotid gland abscesses in which the dosage of antifungal agents is described and along with surgical management via incision and drainage, a successful outcome was reached.5,9
The principles of management of mycotic parotitis include timeous initiation of targeted antifungal therapy. In the majority of cases, high-dose fluconazole was considered first line, and a 78% successful treatment rate has been reported.3-5,8,9
The surgical approach to a fungal parotid abscess should be graduated, where, in the absence of complications, less invasive, ultrasound-guided serial needle aspirations may suffice and will provide the necessary specimens for culture and sensitivity to direct antifungal therapy.6 I&D may be required for source control in the presence of overwhelming sepsis, failed conservative treatment or with large collections with or without extension into the deep neck spaces.6
These two cases described depict the importance of primary management of a fungal parotid abscess via surgical source control and thereafter, culture directed antifungal therapy to avoid resistance. Mycotic infection should be considered as part of the differential in any patient presenting with a parotid abscess, especially in the context of immunosuppression and should be considered in the differential diagnosis.
Our approach was to manage parotid abscesses in two different ways as determined by the general clinical condition of the patient, with both having acceptable and clinically successful outcomes. Serial aspiration may be considered as initial first-line therapy. However, progression to incision and drainage may be considered in order to obtain source control in recalcitrant or complicated infections.6
Conflict of interest
The authors declare no conflict of interest.
Funding source
No funding source to be declared.
Ethical approval
The author/s declare that this submission is in accordance with the principles laid down by the Responsible Research Publication Position Statements as developed at the 2nd World Conference on Research Integrity in Singapore, 2010. Informed verbal consent was obtained from one patient for being included in the case report, the second patient demised and informed verbal consent was obtained from the CEO of the hospital.
ORCID
AK Ogonowski Bizos https://orcid.org/0000-0002-7887-8919
M White https://orcid.org/0000-0003-3432-2086
J Fagan https://orcid.org/0000-0001-7924-7265
V Preterms https://orcid.org/0000-0001-5907-5224
REFERENCES
1. Tsai Y, Yeh C, Chen Y, Chen Y, Huang S. Bilateral parotid abscesses as the initial presentation of strongyloidiasis in the immunocompetent host. Head Neck. 2011;34(1):1051-4. https://doi.org/10.1002/hed.21124. [ Links ]
2. Tan V, Goh B. Parotid abscess: a five-year review - clinical presentation, diagnosis and management. J Laryngol Otol. 2001;121(9):812-9. https://doi.org/10.1011/S0022215106004166. [ Links ]
3. Leibowitz JM, Montone KT, Basu D. Warthin tumour presenting as a fungal abscess in an immunocompetent host: case report and review of the literature. Head Neck. 2010;32(1):133-6. https://doi.org/10.1002/hed.21015. [ Links ]
4. Even-Tov E, Niv A, Kraus M, Nash M. Candida parotitis with abscess formation. Acta Otolaryngol. 2006;126(3):334-6. https://doi.org/10.1080/00016480500388992. [ Links ]
5. Shingde R, Kim T, Givney R, De Waal P, Berry C. Acute parotitis due to Candida glabrata. Infect Dis Clin Pract. 2019;27(6):325-7. https://doi.org/10.1097/IPC.0000000000000784. [ Links ]
6. Scattergood S, Moore S, Prior A, Yusuf G, Sidhu P. Percutaneous drainage of a parotid gland abscess under contrast-enhanced ultrasound guidance: a case report. Ultrasound. 2018;26(3):182-6. https://doi.org/10.1177/1742271X18766705. [ Links ]
7. Pappas P, Rex J, Sobel J, et al. Guidelines for treatment of candidiasis. Clin Infect Dis. 2004;38(2):161-89. https://doi.org/10.1086/380796. [ Links ]
8. Jenks J, Czachor J, Gibbs P, Taylor E. Suppurative parotitis due to Candida glabrata. Infect Dis Clin Pract. 2010;18(3):162-4. https://doi.org/10.1097/IPC.0b013e3181d2ee15. [ Links ]
9. Enache-Angoulvant A, Torti F, Tassart M, et al. Candidal abscess of the parotid gland due to Candida glabrata: report of a case and literature review. Med Mycol. 2010;48(2):402-5. https://doi.org/10.3109/13693780903176503. [ Links ]
 Correspondence:
Correspondence:
Email: ent.nsh@gmail.com














