Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Surgery
On-line version ISSN 2078-5151
Print version ISSN 0038-2361
S. Afr. j. surg. vol.61 n.2 Cape Town 2023
http://dx.doi.org/10.36303/SAJS.3316
SURGICAL ONCOLOGY
The clinicopathological spectrum and treatment outcomes in metastatic colorectal cancer in the KwaZulu-Natal province of South Africa
S Kader; Y Moodley; TE Madiba
Department of Surgery, University of KwaZulu-Natal and Inkosi Albert Luthuli Central Hospital, South Africa
ABSTRACT
BACKGROUND: In high-income countries (HICs) 17-20% of colorectal cancer (CRC) patients have metastatic CRC (mCRC) at the time of diagnosis, of which 10-25% are or become resectable, and a further 4-11% of patients will develop metachronous metastases. The study aimed to establish the prevalence and pattern of metastatic CRC to document treatment outcomes in KwaZulu-Natal (KZN), and to compare results to international norms
METHODS: The study population comprised patients with mCRC presenting between 2000 and 2019. Demographics, primary tumour site, spectrum of metastatic disease and resection rate were assessed
RESULTS: MCRC occurred in 33% of the CRC patient population. Eight hundred and thirty-six patients had metastatic disease, comprising Africans (325, 38.8%), Indians (312, 37.3%), Coloureds (37, 4.4%) and Whites (161, 19.2%). Six hundred and fifty-four patients (79%) had synchronous metastases and 182 patients had metachronous metastases (21%). Single organ metastases occurred in 596 patients (71.2%) (M1A) and multiple organ metastasis occurred in 240 patients (28.7%) (M1B). Metastases occurred in the liver (613), lung (240) and peritoneum (85). Fifty-two patients (6.2%) underwent resection of their metastases
CONCLUSION: The prevalence of stage IV CRC in our setting is at the upper limit of international norms. mCRC occurred in 33%, with similar proportions in all races. Resection rate for metastases is low
Keywords: colorectal cancer, stage IV colorectal cancer, resection, metastases
Introduction
Stage IV colorectal cancer (CRC) is defined as CRC with the presence of distant metastases. Approximately 17-20% or more of patients with CRC have metastatic disease at the time of diagnosis, and a further 4-11% will develop metachronous metastases. The liver is the most common site of metastases but this is dependent on the accuracy and resources to stage these patients.1-4 In 10-25% of patients with metastatic CRC (mCRC), the metastases become resectable following the use of chemotherapy.2 With improving surgical techniques of liver and lung resections in high-income countries (HICs), more patients are becoming candidates for resections.5,6
The public sector in South Africa (SA) is responsible for the care of 80% of the population, while the private sector treats less than 20% of the population.7,8 Brand et al. have shown that in the private healthcare sector of SA, only 7.2% of patients with lung and liver metastases from CRC underwent resection for their metastases.5 For the majority of CRC patients with metastases in the public healthcare setting in KwaZulu-Natal (KZN), there are limited curative options. A significant benefit in median overall survival (OS) has also been shown internationally with palliative systemic treatment.1,8 With the current lack of resources and there being no current public screening initiatives, the auditing of the public health system is necessary to assess the effectiveness of the health services in this domain and compare the rates of resection and the percentage of patients who receive palliative chemotherapy. A comparison of the metastatic resection and palliative chemotherapy rates between SA and the rest of the world would provide important information around the current standard of care within our country and will quantify the level of input required to match world norms.
Patients and methods
Study setting
The study was carried out at the Durban colorectal unit situated at Inkosi Albert Luthuli Central Hospital (IALCH), a tertiary referral hospital in KZN, SA. It houses the colorectal and oncology units, both of which participate in the gastrointestinal cancer multidisciplinary team (MDT). Additional colorectal and oncology units are situated at Addington Hospital (ADH) in Durban and Grey's Hospital (GH) in Pietermaritzburg, both of which are subsidiaries of the main units at IALCH. GH refers only those patients requiring liver resection to IALCH. All patients with CRC cancer, including palliative cases, are discussed at the MDT meeting, which consists of surgeons, oncologists, and radiologists, colorectal, hepatopancreato-biliary and cardio-thoracic teams. Decisions to resect are made in consensus based on resectability and patient fitness.
Patients
The study included all patients with histologically proven CRC extracted from the CRC database entered between 2000 and 2019. Patients with colonic cancers are generally managed surgically at the regional hospitals and are referred to IALCH only after resection unless they present with complicated disease. Patients with rectal cancers are referred to the multidisciplinary clinic before treatment for management decisions. Exceptions are patients who present at the base hospital with tumour-related complications such as obstruction, perforation, and fistula. These patients undergo the emergency procedure for the primary tumour and are referred to the MDT thereafter. Population groups are defined as African, Indian, Coloured and White according to the criteria used by the South African Government. In SA, "Coloured" refers to people of mixed ancestry.9 All patients with stage IV CRC diagnosed on staging CT scan, abdominal ultrasound, MRI or at surgery were included.
Study design
The study is a sub-analysis of prospectively collected data in the CRC registry. The following variables were analysed: age at presentation, gender, race, clinical presentation, site of primary tumour, fixity of the primary tumour, site of metastasis and the management of the primary and secondary tumours. Synchronous metastases were defined as metastases detected at initial diagnosis and/or initial diagnostic work-up, whereas metachronous metastases were considered as those that were detected beyond the time of diagnosis of the primary tumour.10 Our unit policy for imaging surveillance for metachronous lesions is imaging in the case of development of new symptoms or a rise in the serum carcinoembryonic antigen (CEA). M1A was used to describe metastases to one organ and M1B to more than one organs.1
Resectable CRC liver metastases were defined as metastatic liver disease in which a R0 resection can be performed, leaving at least 20-25% of total parenchymal volume with adequate inflow, outflow and biliary drainage.11 Local policy is to give neoadjuvant chemotherapy to patients with potentially resectable and borderline metastases. Resectable lesions are resected up front. Currently, we offer liver resection, portal vein embolisation (PVE) and trans arterial chemo-embolisation (TACE). Patients with unresectable metastases are given palliative chemotherapy or only best supportive care if they are in poor general condition based on their ECOG Score.12 Each patient's management is individualised to their clinical condition and fitness for surgery. Chemotherapy for stage IV disease in our unit is 5-FU-based with oxaliplatin as first-line combination (Folfox regimen) and irinotecan as second line (Folfori regimen), which is used for both neoadjuvant and adjuvant therapy. We do not have access to biological agents. Cycles can vary between six and 12 depending on patient tolerance and compliance.
Statistical analysis
Data were processed with SPSS 11.5 statistical program for Windows. Pairwise comparison was used to compare metastasis extent by age, ethnicity and gender.
Ethical considerations
All patient data was anonymous and confidentiality for participants was maintained.
Results
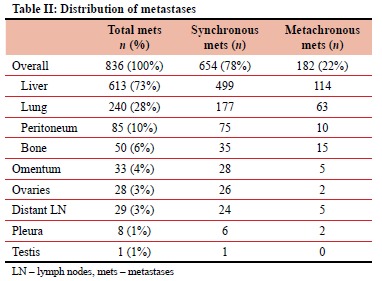
Of the 2 521 patients accrued over the inclusion period (2000-2019), 836 (33%) had stage IV CRC. Six hundred and fifty-four patients had synchronously detected metastases and in 182 (21.7%) metastases were detected later (metachronous metastases). The patient characteristics are shown in Table I. The median age at presentation was 59 (range 48-68) years and there were 438 males with a male-to-female ratio of 1:1. African patients were the youngest at the time of presentation compared to the other population groups, with a median age of 52 years. The sites of the primary tumour were colon (392, 46%) or rectum (440, 54%), giving a colon-to-rectum ratio of 1:1.2. Four patients had synchronous primary colonic and rectal lesions. The 836 patients were sub-staged M1A (596, 71.2%) and M1B (240, 28.8%). Table II shows the distribution of metastases. The liver was the most common target organ (73%), followed by the lungs (28%).

Eighty-three patients received neoadjuvant therapy for their metastases, and 195 received adjuvant chemotherapy for their primary lesion after surgical resection (Table III). One hundred and eighteen patients did not receive chemotherapy for various reasons including poor general condition (11), refusal of treatment (6) and failure to return for treatment (3) and the remainder were lost to follow-up or did not attend their first oncology appointment. The remaining patients (n = 434) received palliative chemotherapy. Eighty-three patients started with neoadjuvant therapy for metastases, but 35 did not respond to therapy and were converted to palliative. A total of 214 patients (25.7%) were lost to follow-up.
Resection of metastases was performed in 52 (6.2%) patients, with liver resections in 36 (4%) patients, oophorectomy in 10 (1%), and lung resection in six (1%) (Figure 1). In the synchronous and metachronous groups 27 (4%) and nine (5%) liver resections were performed, respectively. Two patients with liver metastases received chemo-embolisation, two received microwave ablation and one received radio-frequency ablation. None of these radiology-guided interventions were followed by a liver resection. Lung resections were performed in four patients within the synchronous group and two within the metachronous group. Oophorectomy was performed in 10 patients within the synchronous group.
Discussion
In our study, the anatomical location of the primary tumour was shown to mimic international trends, with the rectum being the most common site.13 MCRC accounted for 33% of patients with CRC, which is higher than the 17-20% reported in the international literature.1,4 The fact that stage IV disease occurred similarly in younger (< 40 years) and older patients (> 40 years) underscores the potential for similar disease progression in young and old presenters, however, this remains controversial and is a subject of debate.14 Males and females had equal distribution of mCRC. The liver accounted for 73% of the overall metastatic burden, which is similar to previous reports of 70%.15 Seventy-one per cent of the patients were staged M1A. The lungs were the second most common site (28%), which is higher than the 10-15% reported in the literature.6 In our series, the most common combination of metastases in M1B patients was liver and lung metastases, which occurred in 122 patients (14%).
The management of stage IV CRC is improving with aggressive surgical resection and better chemotherapeutic agents resulting in improved survival. Approximately 75% of patients with stage IV CRC referred to specialist centres are considered unresectable at presentation prior to the institution of chemotherapy.16 Liver resection offers the chance of potential cure for patients with colorectal liver metastases. The definition of potentially and borderline resectability has yet to be universally defined.17 Some authors use the definition of potentially resectable metastases as R0 resection being compromised as a result of a large tumour burden, but the volume, outflow, inflow and biliary drainage of the future liver remnant (FLR) were considered adequate and the patient would benefit from systemic therapy and restaging.11 Borderline resectable means the possibility of radical resection but, compared to initially resectable, it is considered to be difficult oncologically and technically.17 Only 36 patients (4%) were offered liver resection of metastases in this series, which is below the 25% reported in the international literature and lower than the 10% reported in the private sector in SA.5,18 The reasons for the low liver resection rate in our unit are multi-factorial, including patient refusal for further surgery or chemotherapy, advanced disease, lack of biological agents and high attrition rates. Radio-frequency ablation, microwave ablation and trans-arterial chemo-embolisation for the treatment of liver metastases are available in the state sector in SA, but to a limited extent for selected cases.19
There are no clear criteria for surgical resection pulmonary metastases.20 However, pulmonary resection is regarded as the standard of care for resectable pulmonary metastases based on results of retrospective studies.20 Resection of multiple lung lesions is associated with poor surgical outcomes compared with solitary lesions. Resection of two to four lesions can be considered if the primary tumour has been treated and the patient has sufficient lung function.21 Only 2% of patients with lung metastases were resected, which is much lower than previously reported rates of 21%.21 Resection of solitary lung metastases is associated with low morbidity and mortality and 5-year survival rates of 21-63%.622 Stereotactic radiation and local ablation have been shown to be curative in smaller tumours. Our resection rate is low comparatively because most patients being M1B with simultaneous lung and liver metastases and had multiple rather than solitary lesions precluding them from surgery. Another compounding factor is patient reluctance to undergo further surgery. Currently used chemotherapeutic agents for lung metastases seems to be as effective as in the liver.21
Peritoneal carcinomatosis secondary to CRC occurs in 4-5% of patients with stage IV.23 The peritoneal cavity has been shown to be the only site of metastatic disease in approximately 25% of patients with metastases,13 leading some to postulate that peritoneal carcinomatosis may represent a first site of dissemination in some cases, and is therefore not necessarily indicative of generalised disease.24 Recent data suggest that long-term survival can be achieved using aggressive cytoreductive surgery followed by hyperthermic intraperitoneal chemotherapy (HIPEC) which is used in some centres of excellence.25,26 We currently do not have this facility in our unit.
Only 3% of patients had isolated ovarian metastases which is comparable to the 1-4% reported in the literature.27 Whereas ovarian metastases have been reported to be more common in premenopausal women, with the colon as the site of the primary tumour, in our series it was more commonly seen in postmenopausal women with the rectum as the primary site.28 Resection is associated with fairly low morbidity and may improve quality of life and prolong survival, even in the setting of widespread extra-ovarian metastatic disease.28 Only 38.4% of patients with ovarian metastases in this series underwent oophorectomy, compared to as many as 77% of patients with isolated ovarian metastases in reported series.29
The reasons for the low resection rates in our series, including poor patient compliance regarding follow-up and treatment, delayed presentation, and patient unwillingness to undergo surgical intervention need to be addressed. Dedicated MDT meetings which have been shown to have substantial impact on patient management, need to be established.30 Ideally, all patients should be discussed in an MDT meeting to optimise decision-making.
Access to oncological resources for all patients within a reasonable timeframe to facilitate early diagnosis and management will improve the resection rate. Adequate sub-specialty training in SA needs to be fostered for general surgeons, this will facilitate more skilled surgeons to address the volume of metastatic resections. With an increase in volume of cases, the complexity of cases that can be dealt with will also increase and more patients will be offered resection. The improved combination chemotherapy regimens used in the treatment of patients with advanced CRC can facilitate the downsizing of colorectal liver metastases and render initially unresectable metastases resectable.1,27,28 Access to newer and more effective chemotherapeutic and biological agents has substantially improved survival and quality of life for patients with stage IV disease.33 These agents are not readily available in the public sector in SA.
Our study does have some limitations. The database reflects a single academic institution and the three affiliated tertiary hospitals. Some patients with a diagnosis of CRC may have refused hospital admission and therefore not referred to the three main tertiary hospitals or may have succumbed to their illness prior to referral. Under-diagnosis does therefore remain a possibility. These findings relate to patients in the KZN region and should not be generalised to the rest of SA. The strength of the study is that it is one of the largest gastrointestinal (GI) cancer registries in an African country.
Conclusion
The prevalence of mCRC in our setting is at the upper limit of international norms. Resection rate for metastases in SA is very much lower than international norms. Access to newer chemotherapeutic agents and aggressive metastatic resection need to be encouraged to improve survival. Patient compliance and adherence needs to be improved with efficient accessibility to oncological units. Our local units need further funding and research to improve outcomes.
Conflict of interest
The authors declare no conflict of interest.
Funding source
No funding was required.
Ethical approval
Ethical approval was obtained from the Biomedical Research Ethics Committee of the University of KwaZulu-Natal (Brec Ref.: E198/04).
ORCID
S Kader https://orcid.org/0000-0002-6216-5703
Y Moodley https://orcid.org/0000-0002-4119-1734
TE Madiba https://orcid.org/0000-0002-0155-9143
REFERENCES
1. De Mestier L, Manceau G, Neuzillet C, et al. Primary tumor resection in colorectal cancer with unresectable synchronous metastases: a review. World J Gastrointest Oncol. 2014;6:156. https://doi.org/10.4251/wjgo.v6.i6.156. [ Links ]
2. Adam R, De Gramont A, Figueras J, et al. Hot topic managing synchronous liver metastases from colorectal cancer: a multidisciplinary international consensus of the EGOSLIM (Expert Group on OncoSurgery management of Liver Metastases) group. Cancer Treat Rev. 2015;41:729-41. https://doi.org/10.1016/j.ctrv.2015.06.006. [ Links ]
3. Chuang SC, Su YC, Lu CY, et al. Risk factors for the development of metachronous liver metastasis in colorectal cancer patients after curative resection. World J Surg 2011; 35:424-9. https://doi.org/10.1007/s00268-010-0881-x. [ Links ]
4. Reboux N, Jooste V, Goungounga J, et al. Incidence and survival in synchronous and metachronous liver metastases from colorectal cancer. JAMA Netw Open. 2022;5:e2236666.5. https://doi.org/10.1001/jamanetworkopen.2022.36666. [ Links ]
5. Brand M, Gaylard P, Ramos J. Colorectal cancer in South Africa: an assessment of disease presentation, treatment pathways and 5-year survival. S Afr Med J. 2018;108:118-22. https://doi.org/10.7196/SAMJ.2018.v108i2.12338. [ Links ]
6. Villeneuve PJ, Sundaresan RS. Surgical management of colorectal lung metastasis. Clin Colon Rectal Surg. 2009;22:233-41. https://doi.org/10.1055/s-0029-1242463. [ Links ]
7. Pillay R. Work satisfaction of professional nurses in South Africa: a comparative analysis of the public and private sectors. Hum Resour Health. 2009;7:15. https://doi.org/10.1186/1478-4491-7-15. [ Links ]
8. Rowe K, Moodley K. Patients as consumers of health care in South Africa: the ethical and legal implications. BMC Med Ethics. 2013;14:15. https://doi.org/10.1186/1472-6939-14-15. [ Links ]
9. Myer L, Ehrlich RI, Susser ES. Social epidemiology in South Africa. Hopkins Bloom Sch Public Heal. 2004;26. https://doi.org/10.1093/epirev/mxh004. [ Links ]
10. Engstrand J, Strömberg C, Nilsson H, Freedman J, Jonas E. Synchronous and metachronous liver metastases in patients with colorectal cancer - towards a clinically relevant definition. World J Surg Oncol. 2019;17:1-10. https://doi.org/10.1186/s12957-019-1771-9. [ Links ]
11. Marshall JL. Managing potentially resectable metastatic colon cancer. Gastrointest Cancer Res. 2008;2:S23-26. [ Links ]
12. Crosara Teixeira M, Marques DF, Ferrari AC, et al. The effects of palliative chemotherapy in metastatic colorectal cancer patients with an ecog performance status of 3 and 4. Clin Colorectal Cancer. 2015;14:52-57. https://doi.org/10.1016/j.clcc.2014.09.010. [ Links ]
13. Aslam MI, Kelkar A, Sharpe D, Jameson JS. Ten years experience of managing the primary tumours in patients with stage IV colorectal cancers. Int J Surg. 2010;8:305-13. https://doi.org/10.1016/j.ijsu.2010.03.005. [ Links ]
14. Vatandoust S, Price TJ, Padbury R, et al. Patterns of care and outcomes for young patients (age < 40) with metastatic colorectal cancer (mCRC): findings from a population-based registry. J Clin Oncol. 2014;32:e17584. https://doi.org/10.1200/jco.2014.32.15_suppl.e17584. [ Links ]
15. Kow AWC. Hepatic metastasis from colorectal cancer. J Gastrointest Oncol. 2019;10:1274-98. https://doi.org/10.21037/jgo.2019.08.06. [ Links ]
16. Hernandez CM, Campos RR, Flores DP, Conesa AL, Paricio PP. Prognostic factors after resection of colorectal cancer liver metastases. Cirugía Espanola (English Ed). 2009;85:32-9. https://doi.org/10.1016/S2173-5077(09)70114-0. [ Links ]
17. Kitano Y, Hayashi H, Matsumoto T, et al. Borderline resectable for colorectal liver metastases: Present status and future perspective. World J Gastrointest Surg. 2021;13:756-63. https://doi.org/10.4240/wjgs.v13.i8.756. [ Links ]
18. Nordlinger B, Van Cutsem E, Rougier P, et al. Does chemotherapy prior to liver resection increase the potential for cure in patients with metastatic colorectal cancer? A report from the European Colorectal Metastases Treatment Group. Eur J Cancer. 2007;43:2037-45. https://doi.org/10.1016/j.ejca.2007.07.017. [ Links ]
19. Venkat SR, Mohan PP, Gandhi RT. Colorectal liver metastasis: overview of treatment paradigm highlighting the role of ablation. Am J Roentgenol. 2018;210:883-90. https://doi.org/10.2214/AJR.17.18574. [ Links ]
20. Ogawa H, Yajima T, Sohda M, Shirabe K, Saeki H. Role of surgical resection and its alternative local therapy for pulmonary metastasis of colorectal cancer. Ann Gastroenterol Surg. 2021;5:747-53. https://doi.org/10.1002/ags3.12472. [ Links ]
21. Lumachi F, Chiara GB, Tozzoli R, Del Contea A, Basso SMM. Factors affecting survival in patients with lung metastases from colorectal cancer: a short meta-analysis. Anticancer Res. 2016;36:13-19. [ Links ]
22. Rama N, Monteiro A, Bernardo JE, Eugénio L, Antunes MJ. Lung metastases from colorectal cancer: surgical resection and prognostic factors. Eur J Cardio-Thoracic Surg. 2009;35:444-9. https://doi.org/10.1016/j.ejcts.2008.10.047. [ Links ]
23. Kobayashi H, Kotake K, Sugihara K. Outcomes of surgery without HIPEC for synchronous peritoneal metastasis from colorectal cancer: data from a multi-center registry. Int J Clin Oncol. 2014;19:98-105. https://doi.org/10.1007/s10147-012-0505-6. [ Links ]
24. Franko J, Shi Q, Goldman CD, et al. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of North Central Cancer Treatment Group phase III trials N9741 and N9841. J Clin Oncol. 2012;30:263-7. https://doi.org/10.1200/JCO.2011.37.1039. [ Links ]
25. Elias D, Gilly F, Boutitie F, et al. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric french study. J Clin Oncol. 2010;28:63-8. https://doi.org/10.1200/JCO.2009.23.9285. [ Links ]
26. Yang SY, Kang JH, Kim HS, et al. Status of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in patients with peritoneal carcinomatosis from colorectal cancer. J Gastrointest Oncol. 2019;10:1251-65. https://doi.org/10.21037/jgo.2019.01.36. [ Links ]
27. Al-Agha OM, Nicastri AD. An in-depth look at Krukenberg tumour: an overview. Arch Pathol Lab Med. 2006;130:1725-30. https://doi.org/10.5858/2006-130-1725-AILAKT. [ Links ]
28. Kim DD, Park IJ, Kim HC, Yu CS, Kim JC. Ovarian metastases from colorectal cancer: a clinicopathological analysis of 103 patients. Color Dis. 2009;11:32-38. https://doi.org/10.1111/j.1463-1318.2008.01543.x. [ Links ]
29. Ursem C, Zhou M, Paciorek A, et al. Clinicopathologic characteristics and impact of oophorectomy for ovarian metastases from colorectal cancer. Oncologist. 2020;25:564-71. https://doi.org/10.1634/theoncologist.2019-0282. [ Links ]
30. Kozak VN, Khorana AA, Amarnath S, Glass KE, Kalady MF. Multidisciplinary clinics for colorectal cancer care reduces treatment time. Clin Colorectal Cancer. 2017;16:366-71. https://doi.org/10.1016/jxlcc.2017.03.020. [ Links ]
31. Scartozzi M, Siquini W, Galizia E, et al. The timing of surgery for resectable metachronous liver metastases from colorectal cancer: better sooner than later? A retrospective analysis. Dig Liver Dis. 2011;43:194-8. https://doi.org/10.1016/j.dld.2010.07.003. [ Links ]
32. Terblanche J, Krige JEJ, Bornman PC. Simplified hepatic resection with the use of prolonged vascular inflow occlusion. Arch Surg. 1991;126:298-301. https://doi.org/10.1001/archsurg.1991.01410270038006. [ Links ]
33. McCormack PL, Keam SJ. Bevacizumab: a review of its use in metastatic colorectal cancer. Drugs. 2008;68:487-506. https://doi.org/10.2165/00003495-200868040-00009. [ Links ]
 Correspondence:
Correspondence:
S Kader
Email: shakeelkader2006@gmail.com














